Behold the First Human α-Synuclein CryoEM Fibril Structure
Quick Links
The first high-resolution cryoEM structures of pathological α-synuclein aggregates were unveiled at Tau2020, a new conference held Feb 12 and 13 in Washington, D.C. (see Part 1 of this series). Michel Goedert, MRC Laboratory of Molecular Biology, Cambridge, U.K., presented an assembly that looks quite distinct from the core protofibrils found in tau neurofibrillary tangles or amyloid-β plaques. What’s more, two different protofibrils make up the core of α-synuclein filaments, rendering it asymmetric. Scientists hope this information will finally help them score compounds that will make good ligands for α-synuclein PET imaging, which have thus far eluded them.
- CryoEM solves structure of α-synuclein fibrils from multiple system atrophy.
- Two different fibrils each comprise two different protofibrils.
- Ratio of the fibrils correlated with how long a person had the disease.
Goedert revealed the structure while accepting the Rainwater Prize, a $250,000 award for outstanding innovation in neurodegenerative research. The Rainwater Charitable Foundation co-sponsored the conference. Patrick Hsu, University of California, Berkeley, received a $150,000 innovative early career scientist award for correcting defective tau splicing in MAPT-mutant neurons with CRISPR RNA editing (Oct 2019 news). On March 4, Rainwater and the Alzheimer’s Association announced eight new research grants, totaling nearly $4 million, as part of their Tau Pipeline Enabling Program. The awards go to drug discovery programs at private and academic labs in the U.S. and Europe.

Type I α-Synuclein. Type I α-synuclein fibrils comprise two different protofibrils. PF-IA (yellow) folds from amino acids 14 to 94. Its N-terminus (left) forms a cross β-sheet hairpin that continues into an L shape. Starting at amino acid 45, the C-terminus (right) forms three stacked L shapes. PF-IB (orange) comprises amino acids 21 to 99, and similarly stacks three L-shaped layers at its C-terminus (left). [Courtesy Schweighauser et al., 2020, under Creative Commons license.]
In collaboration with Masato Hasegawa at the Tokyo Metropolitan Institute of Medical Science, Goedert, co-senior author Sjors Scheres from the MRC, and colleagues isolated α-synuclein fibrils from five people who had had multiple system atrophy. MSA is a synucleinopathy that mostly affects glial cells. In his speech, Goedert noted that, as is the case for tau, human brain-derived structures are markedly different than those of recombinant α-synuclein. “This casts doubt on a lot of the work done using recombinant molecules,” he emphasized. “We see again in the case of synuclein that recombinant proteins do not reflect the pathology in the brain.”
In their paper uploaded to bioRxiv on February 6, co-first authors Manuel Schweighauser, Yang Shi, and colleagues describe two different forms of fibril present at different ratios in these five people. The three who had died after nine or ten years with MSA had predominantly Type I fibrils. Type II fibrils predominated in the brains of the other two, who had lived with MSA for 18 and 19 years, respectively. This hints that fibril structure may have something to do with how quickly MSA leads to death, though more evidence is needed to be sure, the authors say.
Complicating matters, the scientists found different fibrils in different brain regions. In one case, Type I fibrils marked the putamen, Type II fibrils the cerebellum. Another case had mostly Type I in the putamen, Type II in the frontal cortex.
The researchers resolved the structure of Type I fibril cores from one patient to a resolution of 2.6 Ångstroms (see image above). One protofibril, PF-IA, encompassed amino acids glycine 14 to phenylalanine 94. PF-IB was slightly shorter, running from lysine 21 to glutamine 99. Both contained extensive β-sheet structure—12 strands in PF-IA and 10 in PF-IB. Both have a β-sheet hairpin at the N terminus and a three-layered, L-shaped β-sheet motif at the C terminus. PF-IA also has a long straight section connecting the two ends; PF-IB does not. The arrangement of the three-layer L differs between the two, particularly in how the innermost layer packs against the middle layer after glycine 86.

Type II α-Synuclein. Protofibril IIA (light purple) adopts almost the same shape as PF-IA (see image above), but features a wider cavity between the inner and central layers of its three-layered C-terminal motif. PF-IIB (dark purple) is 15 amino acids shorter than PF-IB. It comes in two forms. PF-IIB1 forms nearly the identical structure at its C terminus as does PF-1B. PF-IIB2 assumes a slightly different backbone shape. [Courtesy Schweighauser et al., 2020, under Creative Commons license.]
Type II fibrils from one donor were solved to a resolution of 3.1 Ångstroms. PF-IIA resembled PF-IA, extending from glycine 14 to phenylalanine 94 with the same basic folds. However, the three-layered motif at the C-terminus packed together slightly differently, opening a cavity between the inner and central layers near glutamic acid E61. It also has 12 β strands.
PF-IIB turned out to be much smaller than PF-IB. It starts at glycine 36 rather than lysine 21, and has but nine β strands. PF-IIB exists in two distinct forms as well. PF-IIB1 is nearly identical to PF-IB, but PF-IIB2’s backbone is shaped differently between amino acids 81 and 90 (see image above).
A and B Type protofilaments abut to form the Type I and II fibril cores.
What’s With the Cavities?
In a curious nod to tau structures from people with corticobasal degeneration (CBD) and chronic traumatic encephalopathy (CTE), the center of the asymmetrical α-synuclein structure features a small cavity that contains an unknown molecule unattached to the protein (Feb 2020 news).
Schweighauser and colleagues believe this molecule, or molecules, is something other than a protein. It is almost certainly negatively charged, to counter a net positive charge of four lysines that surround the cavity. It may stabilize the structure and even promote assembly, though that remains to be determined. In Type I filaments, the slightly bigger cavity seems to contain additional smaller molecules. Other molecular entities appear on the fringes of the protofilament cores; they may represent extensions of the synuclein polypeptide chain.
Because of the height of the amino acid side chains, and also because the protofibrils tilt away from the fibril axis, each protofibril monomer contacts three monomers in the opposing protofilament. For example, PF-IA’s N-terminus connects to the C-terminus of the PF-IB one rung up the chain, while PF-IA’s C-terminus connects to the N-terminus of the PF-IB one down the chain (see image below).
This is different than in tau filaments. There, each protofilament only contacts its opposing counterpart. The interface between the synuclein monomers is larger, stretching for 25 amino acids, in comparison to at most six amino acids for tau fibrils. “This is bound to have an impact on seeded aggregation, as it results in differences in enthalpic and entropic gains when a new molecule is incorporated into the filament,” the authors wrote.
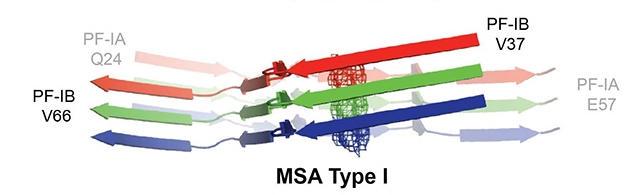
Protofibril Troika. Each synuclein protofilament monomer touches three in the opposing chain. The N terminus of the green PF-IB contacts the C terminus of the faded red PF-IA one up the chain. The C terminus of the green PF-IB binds the N terminus of the faded blue PF-IA one down the chain. [Courtesy Schweighauser et al., 2020, under Creative Commons license.]
This information is fine and good, but does it help scientists understand why mutations in α-synuclein cause disease? Yes. For example, either a glycine-to-aspartic acid mutation at amino acid 51, or an alanine-to-glutamic acid switch at 53 lead to familial Parkinson’s disease. Both residue 51 and 53 lie at an interface between the two protofilaments. The filament structure can accommodate either the aspartic acid or glutamic acid side chains. Each will add one extra negative charge per protofibril, or two for each rung of the fibril structure, reducing the net charge in the cavity by two. This might affect the non-protein mystery molecule in the cavity; this idea, however, requires more work.
Researchers are also banking on cryoEM for clues toward PET imaging ligands. They have sought suitable compounds for years, but failed to find any that bind with high specificity and affinity. Robert Mach, at University of Pennsylvania, Philadelphia, leads a new collaboration to model compounds that might bind known α-synuclein structures. So far, scientists have based their screening on steady-state NMR and cryoEM of recombinant synuclein. But in-silico modelling is only as good as the structural information it uses.
“This is why these new cryoEM structures are so incredibly important,” Mach told Alzforum. “They differ from the NMR and the four to five cryoEM structures of recombinant synuclein solved to date. It does not take a very large change to dramatically affect binding sites,” he said. While some lab-made types contain the three-layered L shape, most are symmetrical, containing only one, or sometimes two identical protofilaments. None of the prior structures have the long N-terminal arms. “We hope the structures of α-synuclein filaments from MSA will help to design PET ligands that are specific for assembled α-synuclein,” Goedert told Alzforum.
What about fibrils from other α-synucleinopathies, such as PD or dementia with Lewy bodies (DLB)? Will they each have their own unique structures, just as different tauopathies do? It seems likely.
Goedert and colleagues tried to solve cryoEM structures for α-synuclein fibrils isolated from three people who had had neuropathologically confirmed DLB. Unlike the MSA fibrils, the DLB ones were thinner and did not twist, as has been previously described (Spillantini et al., 1998; Tarutani et al., 2018). The lack of a twist meant the researchers were unable thus far to determine a three-dimensional structure. However, based on two-dimensional analysis, Goedert and Scheres concluded that DLB and MSA protofibrils are distinct.—Tom Fagan
References
News Citations
- Tau2020: Meeting for Tauopathies Debuts Genetic Variants
- New Funding for Tauopathies
- CryoEM of CBD Tau Suggests Another Unique Protofibril
Paper Citations
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998 Jul 31;251(3):205-8. PubMed.
- Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M. Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol Commun. 2018 Apr 18;6(1):29. PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B, Matsubara T, Tomita T, Ando T, Hasegawa K, Murayama S, Yoshida M, Hasegawa M, Scheres SH, Goedert M. Structures of α-synuclein filaments from multiple system atrophy. bioXriv, February 6, 2020
Annotate
To make an annotation you must Login or Register.

Comments
National Institute on Aging
As the authors indicate, this has important implications for lab modelling of synucleinopathies. It would seem relatively important to be able to show whether the types of assemblies we use in the lab are at all related structurally to those extracted from brain, because processes such as cellular uptake or signaling to immune cells might be different depending on the physical fold the assemblies take.
One other thing that I think would also be helpful to know is how much of each assembly is present in brain at any given time—I think that understanding not just what we put into model systems but also how much will help us have more confidence that our models are as relevant to the human disease as we can possibly make them.
Make a Comment
To make a comment you must login or register.