Alzheimer’s Disease: In the Eye of the Patient?
Quick Links
Could the eyes be a portal by which to check for Alzheimer’s pathology in the brain? Some researchers at the Alzheimer’s Association International Conference 2014, held July 12-17 in Copenhagen, believe so. Two groups presented results about detecting Aβ in the eye. One team reported that Aβ in the retina correlates with plaque buildup in the brain, while another has similar results from the lens. Both propose to develop these biomarkers as early screening tests for Alzheimer’s disease. “PET amyloid imaging and magnetic resonance are expensive,” said David Knopman, Mayo Clinic, Rochester, Minnesota, who was not involved in either study. If they pan out, these developing techniques may prove “simpler, less invasive, and more feasible to use in a primary-care setting as opposed to a large research center.”
Aβ in the Retina
Shaun Frost, from the Commonwealth Scientific and Industrial Research Organization (CSIRO), Perth, Australia, views the retina as an extension of the brain that provides an opportunity to diagnose and track progression of Alzheimer’s. This layer of nervous tissue at the back of the eye has a similar vasculature to the brain and connects directly to it through the optic nerve. Frost presented preliminary results on a test that he says picks up Aβ plaques in the retina. Participants drink a proprietary formulation of curcumin, a component of the spice turmeric. The compound crosses the blood-retinal barrier, binds to amyloid plaques, and fluoresces, so researchers can detect it with a specialized camera developed by collaborator NeuroVision, a company based in Sacramento, California. Aβ plaques appear as fluorescent spots on the retinal scan (see image below). Frost’s colleagues have conducted experiments on postmortem human retinas and reported that curcumin binds to plaques, Frost said (see Koronyo-Hamaoui et al., 2011).
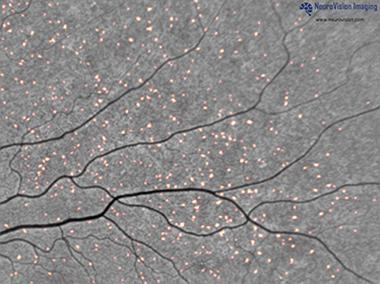
Plaques tagged by curcumin fluoresce in a retinal scan. [Image courtesy of NeuroVision Imaging.]
Frost noted that retinal scans provide 100 to 1,000 times finer resolution than PET scans—down to microns, rather than millimeters. This means researchers can identify individual plaques and monitor their changes.
Thus far, Frost and colleagues have imaged the retinas of 40 of the 200 patients they plan to test from the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL). They were on average 73 years old and had either AD, mild cognitive impairment (MCI), or were healthy. All had undergone a PiB PET scan. In this cross-sectional study, retinal and brain amyloid had a coefficient of correlation of 0.76. Everyone who tested positive in PiB PET, including healthy controls, also showed evidence of plaques in the retina, meaning there were no false negatives. “That’s a crucial component of a screening test for Alzheimer’s because you don’t want to leave any [positive individuals] behind,” said Frost.
However, false positives did occur. Some people whose PiB PET was negative nonetheless tested positive in the retinal scan. Specificity compared to brain imaging was 85 percent. If larger studies bear this out, it might mean that the new technique detects plaque formation prior to PiB PET, suggested Frost. Longitudinal data will be required to find out.
Longitudinal work on this technique is starting now. One MCI patient imaged twice in three months showed a 3 percent increase in retinal plaque, suggesting the technique may eventually help monitor small-scale changes over time, Frost said. Since this method involves no radiation, it could be performed more frequently than PiB PET. It may help determine if candidate drugs remove plaque in clinical trials on a short time scale, said Frost. This technique could also cheaply select people with evidence of AD-related pathology for clinical trials. Some researchers are expressing interest in including these measures in upcoming clinical trials, Frost claimed.
Researchers seem intrigued by the work. One asked if tau could be imaged the same way, and Frost said this has not been addressed yet. Another scientist wondered if fatty deposits called drusen, which associate with age-related macular degeneration, could be confused with Aβ deposits. Frost replied that drusen occur in the macular region in the retina’s center and in its deeper layers, whereas amyloid plaques are peripheral and shallower; therefore the two are easy to differentiate. Jochen Herms, Ludwig Maximilians University, Munich, noted the retina’s known autofluorescence. He talked of having tried time and again in vain to find evidence of Aβ in the retina in mice or in humans, and questioned how researchers can see plaques against background noise. Frost responded that he takes a baseline image before dosing with curcumin and pointed to a publication that reported evidence of retinal amyloid (see Alexandrov et al., 2011).
What about the lens?
Aβ was first reported in postmortem lenses of AD patients more than a decade ago. Lee Goldstein of Boston University, Massachusetts, reported that Aβ40 and Aβ42 accumulated in lens fiber cells (see Goldstein et at al., 2003). Since then, many researchers have tried but failed to reproduce the finding, though others are convinced the Aβ deposits are there and may provide an in vivo biomarker for AD (see May 2013 news story).
Paul Hartung, president and CEO of Cognoptix, Inc. (formerly Neuroptix), in Acton, Massachusetts, presented results from the company’s Phase 2 trial of its Fluorescent Ligand Eye Scanning (FLES) technique, which reportedly detects Aβ in the lens. In FLES, an ointment is applied to the eye the night before a scan. The formulation contains a small fluorophore that diffuses into the eye overnight and binds to Aβ. The next day, a harmless laser delivers quick pulses of light to the eye and detects bound compound by way of its fluorescence.
Hartung said that the method was safe and results correlated with florbetapir amyloid PET scans. In 20 patients with probable AD and 20 age-matched healthy controls, the researchers determined who was positive for amyloid based on a threshold of the fluorescence uptake value. Hartung claimed that with a sensitivity of 85 percent and specificity of 95 percent, lens positivity correlated better to the clinical diagnosis than did brain PiB PET. “We see the potential for this technology to aid in diagnosis and clinical staging of disease,” he said.
Researchers at the meeting asked how the Aβ plaque gets into the lens, which has no blood vessels. Hartung cited evidence that the amyloid precursor protein, secretases, and Aβ-degrading enzymes are present in the lens (see Li et al., 2003); he believes the deposits are likely manufactured in the lens. Someone asked if Cognoptix had correlated what they found with biochemical analysis of lenses removed in surgery or from cadavers. Hartung responded that they had not, but that Goldstein's original study noted the presence of deposits in postmortem lenses.
Scientists were generally skeptical about this technique. Some pointed out that the idea for a lens biomarker has been around for years, yet little has come of it. Others said that since the lens is not directly connected to the brain, it might not reflect brain processes. “The lens is independent of the brain,” Creighton Phelps, National Institute on Aging, Bethesda, Maryland, told Alzforum. “It is difficult to understand how amyloid accumulation in the lens reflects changes in the brain related to Alzheimer’s disease.” Kaj Blennow, University of Gothenburg, Sweden, who chaired the session, agreed, noting that the lens contains no blood vessels, neurons, or nerve terminals. “It is unclear why it would have the same type of pathology as in the brain,” he wrote to Alzforum in an email. “Solid biochemical data showing that there are Aβ deposits in cases with a positive [amyloid imaging] test would be highly desirable.” Others said that if the test works, then conceptual arguments become moot. “If the lens truly accumulates Aβ, then the question is simply how tightly does it mimic brain amyloid levels?” Knopman said. He suggested Hartung and colleagues test the idea in larger, diverse populations.
Many researchers at the conference noted that to their minds, it makes more immediate sense to seek amyloid in the retina, although Blennow maintained that it seems illogical to look for pathology in an organ that is unaffected by symptoms of Alzheimer’s. Frost pointed out that even if a treatment cleared plaque from the brain, it likely would not clear it from the lens, meaning it would not help monitor treatment responses in the brain. The focus of that work is more on differential diagnosis, he said. For his part, Hartung responded that pathologies brought on by macular degeneration or ocular amyloidosis might confound results in the retina.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011 Jan;54 Suppl 1:S204-17. PubMed.
- Alexandrov PN, Pogue A, Bhattacharjee S, Lukiw WJ. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer's disease. Neuroreport. 2011 Aug 24;22(12):623-7. PubMed.
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, Masters CL, Tanzi RE, Chylack LT, Bush AI. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet. 2003 Apr 12;361(9365):1258-65. PubMed.
- Li G, Percontino L, Sun Q, Qazi AS, Frederikse PH. Beta-amyloid secretases and beta-amloid degrading enzyme expression in lens. Mol Vis. 2003 May 1;9:179-83. PubMed.
Further Reading
Papers
- Garcia-Martin ES, Rojas B, Ramirez AI, de Hoz R, Salazar JJ, Yubero R, Gil P, Triviño A, Ramirez JM. Macular thickness as a potential biomarker of mild Alzheimer's disease. Ophthalmology. 2014 May;121(5):1149-1151.e3. Epub 2014 Mar 18 PubMed.
- Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, D'Antonio F, De Vico U, Imbriano L, Campanelli A, De Lena C. Choroidal thinning as a new finding in Alzheimer's disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis. 2014;40(4):907-17. PubMed.
- Tian T, Zhang B, Jia Y, Li Z. Can the lens model provide a biomarker for Alzheimer's disease?. Brain Pathol. 2013 Nov;23(6):696. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Ludwig-Maximilians-Universität Munich
We tried really hard to find any fibrillar Aβ plaques in the retina, both in humans and in various transgenic AD mouse models, but we failed to see what Koronyo et al. 2011 and 2012 had described. In our hands there is no fibrillar Aβ in the retina—which of course may not exclude that there might be occasional patients who do have Aβ plaques in the retina, like those that can be found in the cerebellum of AD patients very occasionally.
Publishing our negative findings was not easy, so those results had to be put into a paper with positive results on the in vivo detection of fibrillar tau in the retina of a transgenic tau mice (P301S) for the first time (Schön et al., 2012).
If you look at the image courtesy of NeuroVision Imaging, those of us familiar with fibrillar Aβ plaques will realize that the proposed curcumin-labelled spots are very small and of similar size. If these are "Aβ-plaques," they are very different from what is seen in the brain of AD patients. But without the retina scan prior to the application of curcumin being shown (which should lack these spots) one may suppose that this is autofluorescence—well known to be present in the retina.
References:
Schön C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, Seeliger MW, Arzberger T, Goedert M, Kretzschmar HA, Schmidt B, Herms J. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7(12):e53547. PubMed.
Make a Comment
To make a comment you must login or register.