How Presenilin Mutations Hobble γ-Secretase Predicts Onset, Progression
Quick Links
The γ-secretase enzyme complex—abandoned as a drug target after candidate molecules proved toxic—is getting another look. New research has correlated the degree to which presenilin mutations affect the enzyme’s propensity to churn out long forms of Aβ with the age at which the mutation causes both Alzheimer's biomarker changes and symptoms.
- Mutations that increase γ-secretase production of Aβ42 and 43 bring on amyloid deposition sooner.
- The degree of γ-secretase dysfunction predicts the age of symptom onset and disease progression.
- Next-generation γ-secretase modulators that reduce production of long Aβ peptides appear to be safe.
The findings were published 26 July in Lancet Neurology. They are good news for scientists developing next-generation γ-secretase modulators. GSM drugs are designed to help the enzyme cut the amyloid precursor protein (APP) into shorter forms of the Aβ peptide, which are less likely to aggregate into amyloid plaques (Jan 2022 news; Feb 2022 news). Recent drug development has focused more on removing amyloid than decreasing its production. Even so, “I think that now [targeting production] will start again, thanks to these modulators,” Bart de Strooper, KU Leuven, Belgium, told Alzforum.
In an editorial accompanying the Lancet Neurology paper, Lucía Chavéz Gutiérrez, also at KU Leuven, Marie-Claude Potier, Institut du Cerveau–Paris Brain Institute, and Harald Steiner, Ludwig-Maximilians–University Munich, wrote that the data “support further investigation of γ-secretase complex modulators as potential Alzheimer’s disease therapies” that could prevent or delay the onset of the familial disease and reduce toxic Aβ levels in sporadic AD.
The role of γ-secretase in amyloid production “is really the best-studied aspect of Alzheimer's,” said de Strooper, who organized a panel on the topic at the Alzheimer's Association International Conference (AAIC) in Philadelphia last month. γ-Secretase has long fascinated researchers because the genes for two of its four component proteins, PSEN1 and PSEN2, are associated with familial Alzheimer's disease. Alzforum’s Mutations database lists more than 300 different PSEN1 variants. Many of them cause AD, but scientists are still studying why individual mutations cause symptoms across such a wide age range. Most mutations do so in a carrier’s 40s and 50s, but the spectrum reaches from the 20s to the 70s.
As scientists dug into these mutations and their links to AD, γ-secretase turned out to be a “funny enzyme,” said the paper’s senior author, Jasmeer Chhatwal, Massachusetts General Hospital, Boston. Over the course of two decades and amidst fierce debates, researchers learned that it holds on to its substrate for a long time and can cut it four or five times before releasing it. Each sequential chop results in a shorter Aβ peptide, and the shorter the peptide, the less likely it is to aggregate into plaques.
This suggested that mutations that weaken γ-secretase’s grip on its substrate allow long Aβ peptides to be released before the enzyme is finished with them. While studying 25 PSEN1 mutations, Chavéz Gutiérrez and her colleagues had found previously that the ratio of short to long Aβ peptides produced in each person’s cells predicted how early they would develop the disease (Petit et al., 2022; Apr 2022 news).
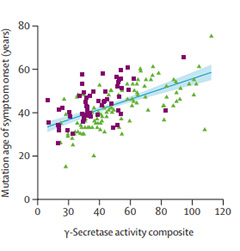
GSC Predicts Onset. A PSEN1 mutation’s γ-secretase composite (GSC) score correlates with its age of symptom onset. Lower scores mean earlier symptoms. Purple squares represent variants in the DIAN observational cohort, green triangles do not.
To study this in a larger group, lead author Stephanie Schultz and colleagues turned to the Dominantly Inherited Alzheimer's Network observational study (DIAN-OBS), which has been collecting clinical and biomarker data from people with PSEN and APP mutations for 15 years (Nov 2008 conference news, Daniels et al., 2024). They identified 190 people with one of 56 different PSEN1 mutations, put each mutation into otherwise identical cultured cells, and compared directly how each variant processed APP, without interference from the rest of each carrier’s genome.
Following a system developed by Chavéz Gutiérrez’s group, Chhatwal’s lab measured the ratio of short to long Aβ peptides produced by each PSEN1 variant. This served as a proxy for each enzyme’s activity level, allowing them to then compute a ratio known as the γ-secretase composite (GSC) between the variant’s activity and the wild-type PSEN1 activity level. While the variants all produced similar total amounts of Aβ, more active γ-secretase variants produced more short peptides.
When the scientists compared these in vitro ratios to each person’s clinical profile and family history, they found a direct relationship between the GSC and age of symptom onset, as well as how fast the disease would progress. Mutations with GSC ratios below 1 brought on symptoms sooner, and their carriers had greater biomarker changes such as CSF phospho-tau and Aβ42/40 ratios.
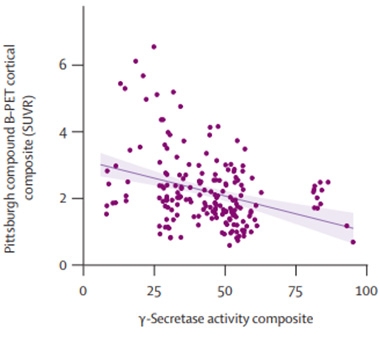
Shorter Is Better. γ-Secretase enzyme variants with low GSCs cleave their APP substrates fewer times, spewing longer Aβ peptides and leading to more amyloid deposition.
The researchers confirmed this trend with 105 more variants described in people not enrolled in DIAN. “It seems like the enzyme function itself is shaping the clinical and biomarker course of the disease,” Chhatwal said.
The same correlation appears to occur with PSEN2 mutations. In a preprint posted to bioRxiv on June 28, Chhatwal’s group looked at 74 PSEN2 variants and found that γ-secretase’s activity level similarly predicted age of onset, although carriers tended to develop Alzheimer's more than 20 years later than those with equivalent PSEN1 mutations (Liu et al., 2024). Chavéz Gutiérrez, who presented her similar findings on PSEN2 in a smaller study at AAIC, told Alzforum this suggests PSEN1 is the more important contributor to toxic Aβ production.
The GSC screening method could help scientists determine which of the many Alzheimer’s-associated PSEN mutations are actually pathogenic. “We think we could almost recharacterize [some variants’] pathogenicity based on the cell-based measure,” Schultz said. The researchers plan to put all their data online for other scientists to use. “We see it as being a resource for researchers who study autosomal-dominant Alzheimer's disease,” she said.
That a given mutation’s GSC predicts age at onset could help with estimating the prognosis of individual mutation carriers. It could also help scientists make the call of whether a newly discovered mutation is likely to be pathogenic. “I think [this method] provides a tool to do fairly fast screening of a lot of different mutations,” Chhatwal says.
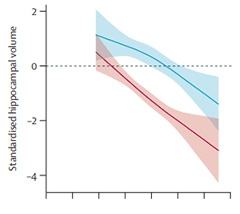
More Shrinkage. People whose PSEN1 variants score lowest on the GSCs (red) have earlier and faster hippocampal atrophy than those in the highest tertile (blue).
“This is not only smoke, this is the gun and everything,” de Strooper said. He added the tight correlation between γ-secretase activity and age of symptom onset shows that—at least in dominantly inherited Alzheimer's—amyloid production is enough to cause the disease. “From a scientific point of view, [γ-secretase modulation] is really the best path to do something about amyloid,” he said.
DIAN trials codirector Eric McDade, Washington University, St. Louis, said the paper “captures all the data we've been collecting in the DIAN study for years.” He said that understanding the gradient of Aβ37 to Aβ43 production could allow scientists to test different drugs or doses in people with different PSEN variants and monitor a given drug’s effect on the ratio of long and short Aβ peptides.
These treatments could start decades before a person’s disease is expected to develop. “If [long Aβ peptides] are not produced, then they will not accumulate, even if amyloid clearance gets impaired during aging,” Chavéz Gutiérrez said. The DIAN trials unit has started a primary prevention trial, where drugs will be evaluated for their ability to head off amyloid deposition in mutation carriers (Aug 2024 conference news).
Getting more drugmakers onboard could be difficult. For about a decade, several companies developed γ-secretase inhibitor drugs, but toxicity ended those trials, likely because blocking γ-secretase prevents it from cutting its dozens of other substrates (Dec 2012 news; Aug 2010 news). A first generation of γ-secretase modulators—which do not block the enzyme but shift its second, processive cleavage toward spitting out more Aβ37 and 38 and less Aβ42 and 43—ran into different toxicity in Phase 1 (Aug 2008 conference news; Dec 2008 conference news; Apr 2011 conference news, EVP-0962, PF-06648671).
Despite all that, “a couple of groups continued doing the hardcore basic research trying to understand [γ-secretase modulation],” de Strooper said.
Then, F. Hoffmann-La Roche, Basel, Switzerland, surprised many last year when it moved a new GSM into Phase 1, where so far it appears safe. Called RG6289, it does not seem to interfere with the enzyme’s ability to process other substrates (Nov 2023 conference news). RG6289 seems to stabilize the bond between γ-secretase and its APP-derived substrate C99, causing the enzyme to cut it into even smaller Aβ fragments that don’t generally occur in nature.
At AAIC, Roche’s Irene Gerlach presented data showing that the drug lowered the concentration of long forms of Aβ by up to 70 percent and increased those of short forms in plasma samples from healthy volunteers in a Phase 1b trial. The company is now testing the drug in 245 people at risk of Alzheimer's or in the disease’s prodromal stage, tracking Aβ ratios and biomarkers such as p-tau for 18 months. Gerlach said these Phase 2 results are expected soon.
Other researchers have developed GSMs that they plan to test in clinical trials beginning next year. At AAIC, Kevin Rynearson, University of California, San Diego, presented preclinical data on compounds being developed by Acta Pharmaceuticals.
De Strooper says the data so far is promising. “I hope in the future we will get a little bit more risk-taking in this field and that we can do a prevention trial,” he said, adding that it will take many years to prove that GSMs can prevent the disease.
Even if they do, McDade added, they may only weakly affect symptomatic AD, where plaques have formed years ago. GSMs may need to be tested in combination with anti-amyloid immunotherapies, or as maintenance therapies once immunotherapy has removed most plaques.—Sara Reardon
Sara Reardon is a freelance writer in Bozeman, Montana.
References
News Citations
- Does More Aβ38 Mean Less Cognitive Decline in Alzheimer’s?
- “Frustrated Oligomers” Slow Aggregation of Aβ42
- Ratio of Short to Long Aβ Peptides: Better Handle on Alzheimer's than Aβ42/40?
- DIAN: Registry for eFAD to Chart Alzheimer’s Preclinical Decade
- First Success Stories From Alzheimer’s Secondary Prevention Trial
- Drug Company Halts Development of γ-Secretase Inhibitor Avagacestat
- Lilly Halts IDENTITY Trials as Patients Worsen on Secretase Inhibitor
- Chicago: Flurizan Postmortem
- Eibsee: Keynote on Anti-amyloid Drugs, Prevention
- Barcelona: Allosteric γ Modulation Moves Toward Clinic
- Second-Generation γ-Secretase Modulator Heads to Phase 2
Therapeutics Citations
Paper Citations
- Petit D, Fernández SG, Zoltowska KM, Enzlein T, Ryan NS, O'Connor A, Szaruga M, Hill E, Vandenberghe R, Fox NC, Chávez-Gutiérrez L. Aβ profiles generated by Alzheimer's disease causing PSEN1 variants determine the pathogenicity of the mutation and predict age at disease onset. Mol Psychiatry. 2022 Jun;27(6):2821-2832. Epub 2022 Apr 1 PubMed.
- Liu L, Schultz S, Saba A, Yang H-S, Li A, Selkoe D, Chhatwal J. The pathogenicity of PSEN2 variants is tied to Aβ production and homology to PSEN1. 2024 Jun 28 10.1101/2024.06.22.600217 (version 1) bioRxiv.
Other Citations
External Citations
Further Reading
Primary Papers
- Schultz SA, Liu L, Schultz AP, Fitzpatrick CD, Levin R, Bellier JP, Shirzadi Z, Joseph-Mathurin N, Chen CD, Benzinger TL, Day GS, Farlow MR, Gordon BA, Hassenstab JJ, Jack CR Jr, Jucker M, Karch CM, Lee JH, Levin J, Perrin RJ, Schofield PR, Xiong C, Johnson KA, McDade E, Bateman RJ, Sperling RA, Selkoe DJ, Chhatwal JP, Dominantly Inherited Alzheimer Network. γ-Secretase activity, clinical features, and biomarkers of autosomal dominant Alzheimer's disease: cross-sectional and longitudinal analysis of the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet Neurol. 2024 Sep;23(9):913-924. Epub 2024 Jul 26 PubMed.
- Potier MC, Steiner H, Chávez-Gutiérrez L. Amyloid β, γ-secretase, and familial Alzheimer's disease. Lancet Neurol. 2024 Sep;23(9):852-853. Epub 2024 Jul 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.