Traumatic Brain Injury—Focus on Heterogeneity, Secondary Damage
Quick Links
Brain injuries often precipitate neurodegeneration, but researchers still know little about the exact mechanisms involved, much less how to treat it. Complicating matters, traumatic brain injury (TBI) can take different forms depending on the nature of the injury, the brain region affected, and how many hits to the head were involved. In recent years, researchers have developed animal models to study these varied types of brain injury. At the Society for Neuroscience annual meeting in Chicago October 17-21, speakers highlighted the distinct pathologies involved in particular forms of TBI, including tau accumulation and axonal injury. Several talks emphasized that the clinical effects of brain injuries vary depending on characteristics such as sex, age, genotype, history, and perhaps even diet. With such a heterogeneous condition, finding treatments is especially challenging. Nonetheless, several researchers described pharmaceutical interventions that preserved neurons and cognition in rodent models when administered soon after injury, hinting that secondary damage can be prevented. Overall, the picture that emerged was of a field still in its infancy, where researchers are just beginning to pin down basic mechanisms by studying a range of animal models.
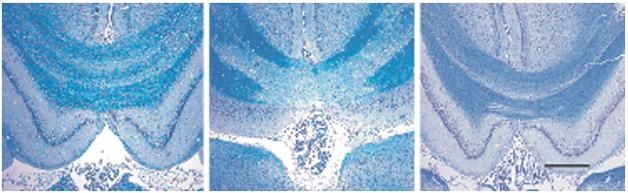
Myelin Protection. In rats that suffer a brain injury, myelin in the corpus callosum deteriorates (middle) compared to that in controls (left), but treatment with a combination of minocycline and N-acetylcysteine preserves it (right). [Courtesy of Haber et al., 2015, submitted.]
Epidemiological studies indicate that head injury confers a roughly 60 percent increased risk of dementia (see Aug 2012 news; Jun 2014 news). But what type of dementia? Some evidence suggests that a single severe blow to the head pumps up amyloid production, and may predispose people to Alzheimer’s (see Nov 2013 news; Jan 2014 news). On the other hand, repetitive but milder injuries, such as those suffered in contact sports or from proximity to explosions in the military, trigger a progressive tauopathy known as chronic traumatic encephalopathy (CTE) (see May 2012 news; Nov 2012 news series; Mar 2014 news). In particular, these repetitive injuries seem to shred fragile axonal connections (see Jan 2013 news).
Many Mechanisms Underlie TBI
Because of this high-profile CTE research, head injuries became synonymous with tauopathies in many researchers’ minds, noted Fiona Crawford of the Roskamp Institute in Sarasota, Florida. However, she and other speakers stressed that TBI is a diverse condition, and its study requires many different animal models of injury. Researchers have now developed protocols that mimic single severe blows to the head, repetitive mild impacts, and explosive blasts, among others. These models vary widely in how much tau they accumulate, as well as in other features, suggesting they may capture the heterogeneity of the syndrome. However, researchers at SfN noted many points of convergence between different forms of injury as well.
Many researchers use a controlled-cortical-impact model, in which a piston strikes either the skull or exposed brain of a rodent. Crawford’s group found that mice that received five mild, closed-head controlled cortical impacts developed neuroinflammation and axon damage along with progressive motor and cognitive defects, but no changes in tau (see Mouzon et al., 2012; Mouzon et al., 2014). Apparently, injured brains do not need to develop tau pathology to experience long-lasting, progressive damage. “It’s not all about tau,” she concluded.
Nonetheless, to study the role of tau in CTE, Crawford developed a protocol to induce chronic TBI. She exposed mice that expressed wild-type human tau to two head injuries per week for two to three months, mimicking an athlete exposed to frequent concussions. In this model, both total tau and phosphorylated tau rose, persisting for at least three months after injury, she reported. In addition, neuroinflammation spiked, cerebral blood flow dropped, and the animals had trouble learning. These mice may model CTE-like degeneration, she suggested.
TBI Beyond Tau
Overall, few of the talks at SfN focused on tau, instead highlighting other aspects of brain damage. Michal Vascak in John Povlishock’s group at Virginia Commonwealth University, Richmond, detailed what happens to axons after brain injury. Vascak used a model of mild TBI in which a device rapidly injects a small volume of saline into a mouse brain, subjecting cells to a fluid concussion wave. This does not cause brain lesions or hemorrhages, but a diffuse, widespread axonal injury ensues as fragile neuronal connections twist and shear around the injury site. Moreover, even those axons that do not break may be affected, Vascak said. He reported that two days after injury, intact axons did not fire properly. He wondered if this might be due to changes in the axon initial segments, where action potentials are generated.
To get a closer look at those segments, Vascak used confocal microscopy to image individual uninjured axons in postmortem mouse brain two days after injury. Using specific markers to identify the ends of initial segments, he found that the distal end had shrunk by about 2 μm. Since this end triggers action potentials, the change would alter neuronal firing properties, and that in turn might affect overall network excitability, Vascak suggested. The data demonstrate that TBI can affect the properties of even intact axons.
How does this happen? Perhaps disconnection of upstream neurons caused by the TBI shifts axon initial segments to a malleable, dynamic state, Vascak hypothesized. This might allow a localized injury to have widespread effects on brain function, as broken axons lead to changes in downstream neurons, which then affect neuronal activity even further downstream. The data jibe with a similar finding of shortened axon initial segments in a blast model of traumatic brain injury (see Baalman et al., 2013). There is precedent for axon initial segment plasticity. They change in length and position in response to alterations in neural activity (see Grubb et al., 2011).
In addition to axonal damage, brain injuries are marked by the pathogenic accumulation of proteins, such as Aβ and TDP-43 in addition to tau. Chinmoy Sarkar, who works in the lab of Marta Lipinski at the University of Maryland School of Medicine, Baltimore, proposed a single mechanism that could explain all these pathologies. The researchers previously reported that a controlled cortical impact disrupted autophagy in cortical and hippocampal neurons. Lysosomes lost the ability to chew up unwanted proteins, and autophagosomes accumulated (see Sarkar et al., 2014). At SfN, Sarkar reported that a rise in cytosolic phospholipase A2 (cPLA2) a few hours after brain injury disrupts autophagy. The phospholipase breaks down lipids in lysosomal membranes, allowing proteases such as cathepsin D to leak out into the cytosol. This limits proteolysis in lysosomes, creating an autophagy roadblock. Treating mice with an inhibitor of cPLA2 after TBI prevented the accumulation of autophagosomes, he said.
Same Injury, Different Outcomes
Even as speakers detailed the multiple things that can go wrong in injured brains, they also stressed specific effects that depend on the characteristics of the animal. In an SfN press conference, Ramesh Raghupathi of Drexel University College of Medicine, Philadelphia, reported that the brains of males and females respond differently to TBI. When mice were subjected to a forced-swim test one to two months after a mild brain injury, males gave up swimming sooner than females. This is typically interpreted as a sign of depression. In addition, dopaminergic transmission fell in the males, another sign they were depressed since dopamine increases the sense of well-being. Females, on the other hand, reacted more strongly than males to a light touch on the face, suggesting they felt more pain. Both depression and headache are common symptoms in people in the months after brain injury. It is not yet clear what determines these sex differences, Raghupathi said.
Age is another crucial factor, with children often having worse outcomes after brain injury, including more post-traumatic seizures than adults (see Arango et al., 2012). At the press conference, Trent Anderson of the University of Arizona in Phoenix reported that three-week-old mice, but not adults, developed epileptiform brain activity after a single moderate injury to the exposed brain (see Nichols et al., 2015). In the brains of these pups, Anderson saw an 80 percent drop in levels of parvalbumin, which marks a subclass of inhibitory interneuron. The neurons themselves did not die, but they changed in shape and size, suggesting they might be disconnecting from the network, Anderson said. The removal of these inhibitory neurons likely amps up overall network excitability. This may explain the greater susceptibility of children to seizures after brain injury, he speculated.
Genotype can also be important. Carriers of the ApoE4 allele have up to 10-fold higher risk of dementia after brain injury, for example (see Mayeux et al., 1995; Zhou et al., 2008; Zeng et al., 2014). At SfN, Crawford reported data from a small study comparing mice that carried the human ApoE4 or ApoE3 alleles. The E4 carriers produced significantly more phosphorylated tau after injury, she said. Crawford also described preliminary data suggesting that mice eating a high-fat, high-carb “Western diet” fare worse after TBI than those consuming healthy, low-fat chow.
Could life experiences also prime the brain to be vulnerable to TBI? Sydney Candy, working in Quentin Pittman and Michael Esser’s labs at the University of Calgary, Canada, found that to be the case. She injected 10-day-old rats with either saline or the inflammatory agent LPS to mimic an early life infection. Twenty days later, the rats received a mild controlled cortical impact brain injury. Rats that had been primed with LPS performed more poorly on novel-object-recognition tests, failed sooner in the forced-swim test, and spent more time in the center of open areas in their cages than did control-injected littermates. In control rats, cytokine levels surged after TBI, but in the LPS-primed animals, they remained at wild-type levels, suggesting failure of an immune response. The data hint that prior neuroinflammation could make the brain more vulnerable to the effects of injury, Candy said.
Neuroprotective Strategies
With such a diverse array of injuries and effects, what are the options for treating TBI? Researchers at SfN suggested targeting neuroinflammation and oxidative damage, which are common after brain injury. Michael Ayo Sangobowale in Peter Bergold’s lab at SUNY Downstate Medical Center, Brooklyn, reported that a combination of the antibiotic minocycline and the modified amino acid N-acetylcysteine (NAC) might help. Both of these drugs have antioxidant and anti-inflammatory actions, and also modify gene expression; it is not clear which of these actions provides the therapeutic benefit, Bergold wrote to Alzforum. Previously, Bergold and colleagues reported that the two drugs together better preserved memory than either alone when given to rats one hour after a mild injury to the exposed brain. The combination prevented neuroinflammation and preserved the myelin sheath around axons (see image above; Abdel Baki et al., 2010; Haber et al., 2013). At SfN, Sangobowale extended this data, presenting evidence that the drug combo protects myelination even when given 12 hours after the initial injury. He is currently investigating how long the therapeutic window lasts.
In addition, Sangobowale reported that rats dosed 12 hours after injury performed better than untreated controls on an active place-avoidance test, in which animals have to learn a pattern of cues in order to avoid a spot where they will receive an electric shock. Treatment 24 hours after injury did not improve performance on this test, but did help rats do better in a Barnes maze, in which animals have to escape down a hole in a set location. Active place avoidance demands a higher level of cognition than the Barnes maze, Bergold wrote to Alzforum. “Therapeutic windows may differ depending upon what outcome measure is being assessed,” he noted. But overall, the take-home was that early treatment has better outcomes.
Many groups are interested in the potential of hyperbaric oxygen therapy to treat the injured brain. In this approach, patients breathe 100 percent oxygen at high pressure for a short period each day. The extra oxygen is believed to nurture and preserve injured neurons, but small clinical trials have yielded mixed results, with one review concluding that more data are needed to determine what type of injuries will benefit from this approach (see Bennett et al., 2012). At SfN, Chaim Pick of Tel Aviv University, Israel, reported that the therapy prevented cognitive decline in mice. One week after receiving a blow to the side of the head, control mice learned poorly in a Y-maze and failed to recognize novel objects as new. However, mice that received hyperbaric oxygen therapy for one hour per day, starting three hours after injury and repeated for four consecutive days, learned as well as wild-types. Injured controls had lost about 20 percent of their neurons and 40 percent of their myelin, while displaying 25 percent more reactive astrocytes. The brains of treated mice, on the other hand, looked like wild-types. The results provide clues as to how oxygen treatment may help damaged brains, the authors suggested.
Bruce Citron at Bay Pines VA Healthcare System, Florida, highlighted a key role for antioxidants. Previously, he had reported that expression of the transcription factor Nrf2, which turns on antioxidants and chaperones, ramped up after a closed-head injury in mice. Treatment with an activator of Nrf2 improved memory in the injured animals, suggesting that Nrf2 upregulation represents a protective response to injury (see Saykally et al., 2012). At SfN, Citron extended these data to a blast-injury model developed by Pick. In collaboration with Jessica Chang at Bay Pines, Citron exposed mice to a pressure wave from an explosive blast to simulate injuries experienced by military service members. As in the impact model, Nrf2 expression soared. Mice treated with an activator of Nrf2 once daily starting 30 minutes after a blast injury expressed less amyloid precursor protein and neurodegenerative markers than controls, Citron said. “There seem to be common themes as well as differences between different types of injuries,” he wrote to Alzforum. Citron is looking for additional ways to modify gene expression after TBI to protect the brain, he added.
Boost in calcium flux after the initial insult might also contribute to pathology, said Rachelle Dugue of SUNY Downstate Medical Center. Dugue works with Douglas Ling at SUNY and Jeffrey Goodman at The Institute for Basic Research in Developmental Disabilities, Staten Island, New York. Increased calcium activates the protease calpain, which cleaves numerous substrates and precipitates neurodegeneration, Dugue said. She evaluated the ability of a new calpain inhibitor, ALA-1.0, to prevent this. ALA-1.0 consists of the known calpain inhibitor leupeptin linked to the anti-epileptic drug pregabalin, which helps the whole compound to cross the blood-brain barrier. When Dugue gave rats 80 mg/kg ALA1.0 immediately after a controlled cortical impact, then examined them two days later, 40 percent fewer neurons degenerated than in controls. In ongoing work, Dugue will determine the therapeutic window for ALA-1.0, and whether the drug also preserves learning and memory.
While overall the presentations at SfN showcased a fledgling field, researchers were energized by the progress made thus far, by the many opportunities for future study, and by the potential to turn some of the discoveries into therapeutic interventions. In particular, scientists agreed that the ability to model the different types of brain injury has helped propel the field forward.—Madolyn Bowman Rogers
References
News Citations
- New AlzRisk Analysis: Brain Injury Promotes Dementia, But Is It AD?
- Brain Injury Boosts Dementia Risk
- Imaging Reveals Amyloid Up To a Year After Traumatic Brain Injury
- Does a Blow to the Head Mean More Amyloid Down the Road?
- Blast Anatomy—Chronic Traumatic Encephalopathy in Military Vets
- Meet the New Progressive Tauopathy: CTE in Athletes, Soldiers
- For Hockey Players, Brain Damage Can Be Measured in Blood
- In Former Footballers, MRI Links Cognitive Problems to Axon Damage
Paper Citations
- Mouzon BC, Chaytow H, Crynen G, Bachmeier C, Stewart JE, Mullan M, Stewart W, Crawford FC. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma. 2012 Aug 17; PubMed.
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol. 2014 Feb;75(2):241-54. Epub 2014 Feb 20 PubMed.
- Baalman KL, Cotton RJ, Rasband SN, Rasband MN. Blast wave exposure impairs memory and decreases axon initial segment length. J Neurotrauma. 2013 May 1;30(9):741-51. Epub 2013 Feb 6 PubMed.
- Grubb MS, Shu Y, Kuba H, Rasband MN, Wimmer VC, Bender KJ. Short- and long-term plasticity at the axon initial segment. J Neurosci. 2011 Nov 9;31(45):16049-55. PubMed.
- Arango JI, Deibert CP, Brown D, Bell M, Dvorchik I, Adelson PD. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst. 2012 Nov;28(11):1925-9. Epub 2012 Jul 28 PubMed.
- Nichols J, Perez R, Wu C, Adelson PD, Anderson T. Traumatic brain injury induces rapid enhancement of cortical excitability in juvenile rats. CNS Neurosci Ther. 2015 Feb;21(2):193-203. Epub 2014 Dec 5 PubMed.
- Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995 Mar;45(3 Pt 1):555-7. PubMed.
- Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008 Apr;25(4):279-90. PubMed.
- Zeng S, Jiang JX, Xu MH, Xu LS, Shen GJ, Zhang AQ, Wang XH. Prognostic value of apolipoprotein E epsilon4 allele in patients with traumatic brain injury: a meta-analysis and meta-regression. Genet Test Mol Biomarkers. 2014 Mar;18(3):202-10. Epub 2014 Jan 29 PubMed.
- Abdel Baki SG, Schwab B, Haber M, Fenton AA, Bergold PJ. Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS One. 2010 Aug 31;5(8):e12490. PubMed.
- Haber M, Abdel Baki SG, Grin'kina NM, Irizarry R, Ershova A, Orsi S, Grill RJ, Dash P, Bergold PJ. Minocycline plus N-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp Neurol. 2013 Nov;249:169-77. Epub 2013 Sep 10 PubMed.
- Bennett MH, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012 Dec 12;12:CD004609. PubMed.
- Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, Citron BA. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience. 2012 Oct 25;223:305-14. Epub 2012 Aug 10 PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Quietmind Foundation
Our current work with monitoring the EEG activity of TBI patients has shown that the increase in slow-wave and decreased-faster-frequency amplitudes corresponds to the biomarker results outlined in this report. We have also found that exposing TBI patients to both brief transcranial near-infrared (1060-1080 nm) light stimulation and operantly conditioning abnormal EEG amplitude and connectivity measures results in significant, lasting symptom remediation. The current studies with Alzheimer's and Parkinson's cognitive and motor planning symptoms suggest similar outcomes.
Make a Comment
To make a comment you must login or register.