Barfing Up Balls of Trouble Keeps Some Neurons Healthy
Quick Links
Throwing up bad clams is no picnic, but it makes people feel better afterward. Neurons seem to know that, too. Researchers in the lab of Monica Driscoll at Rutgers, The State University of New Jersey in Piscataway, and elsewhere, have stumbled on a dramatic stunt by which neurons in C. elegans relieve themselves of intracellular garbage. As reported in the February 9 Nature, neurons pack damaged mitochondria and aggregated proteins into giant vesicles the researchers call exophers. The neurons then hurl these bundles of detritus out into the extracellular space. This is no Hail Mary pass on the part of a sick or dying neuron. On the contrary, the neurons seem just fine afterwards, thank you very much. Even rejuvenated.
The researchers have yet to determine whether mammalian cells make exophers. If they did, that could radically change scientists’ understanding of neuronal metabolism. It could also have implications for a range of diseases including Alzheimer’s, said Driscoll. “We speculate that this is a whole new way of managing trash that we didn’t know about. If it is going on in humans, this could open up a new set of targets for manipulation in neurodegenerative disease.”
Let it all out: A neuron labeled with aggregating florescent mCherry (white) expels much of the protein in a bright exopher, leaving a relatively dim soma behind. [Melentijevic et al., 2017. Nature.]
First author Ilija Melentijevic made the initial discovery. While studying the dendritic branching patterns of aging, fluorescently labeled C. elegans neurons, he noticed occasional bright blobs floating outside the cells. Over two years, he counted and monitored their production and degradation in single still images. Finally, he decided to catch one mid-formation. He spent nights in the lab, taking microscopic images of the same worms every 15 minutes. Eventually, he captured a neuron forming a clear bulge, which over the course of 90 minutes turned into a vesicle as big as the soma—and then pulled away from the neuron. Before pinching off completely, the exopher appeared tethered by a thread-like tube through which the neuron kept filling it. What in the world was going on? Melentijevic strung these images together into a time-lapsed video and played it for Driscoll the next day (see above). “I was stunned,” she said. Convinced that this was important, the lab began figuring out what it meant.
What was inside these exophers? Why did they form? To make sure they weren’t witnessing cell division, the researchers checked the exophers for DNA and found none (see image below). Rather, the membrane-bound vesicles held mitochondria, lysosomes, and aggregation-prone proteins. At up to 7.8 μm wide, they have up to 100 times the girth of exosomes—0.1 μm wide or smaller vesicles of cytoplasmic material that function in extracellular signaling (Dec 2014 news).
Several types of neuron made exophers, including sensory and dopaminergic ones. Neurons that belched one out appeared healthier than those that held their contents in. For example, worms that express a toxic huntingtin protein with a 128 polyglutamine repeat (Htt-Q128) are less responsive to touch, as are their cultured neurons. But neurons from those worms responded normally if they had expelled an exopher.
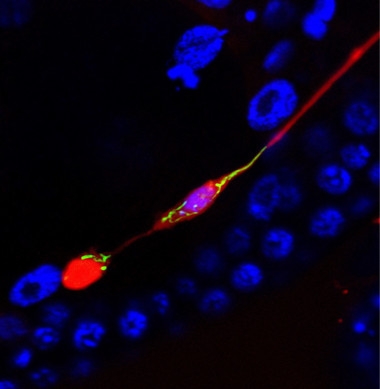
Trash Day.
An exopher (red blob, lower left) pulls aggregating mCherry and mitochondria (green) away from the neuronal cell body (purple, middle of image). A thin fiber still separates them, before the exopher pinches off. Nuclear marker (blue) does not stain the exopher. [Courtesy of Ilija Melentijevic and Meghan Arnold.]
Interestingly, neurons seemed to actively sort harmful proteins and damaged organelles into these vesicles. Worms that expressed Htt-Q128 concentrated the aggregating protein into the exophers. Other neurons that expressed both an aggregating form of mCherry and non-aggregating green fluorescent protein packed in mostly the former. MitoROGFP, a marker of damaged mitochondria, indicated most organelles in exophers were damaged. “That told us this isn’t just a random migration of bulk cellular contents out of the cell,” said Melentijevic. “There’s a specific mechanism the cell uses to target certain undesirable components.” In addition, worm strains that expressed aggregating proteins—including Aβ42—or worms treated with chemicals that damage mitochondria, produced more exophers than controls.
The researchers also found that if other proteostatic pathways became clogged, exopher production picked up. For instance, when the scientists hobbled the activity of chaperone-mediated protein folding, autophagy, or the proteasome, neurons expelled more exophers. This suggests exopher production works as a backup when classical proteostasis pathways can’t keep up with neurotoxic aggregates or organelles, the scientists claim. Exopher production also peaked twice during the lifetime of these worms: first when they were three days old, and again when they neared old age, day 10. In between, they extruded few exophers. Exophers may help remove developmental debris in young adulthood, and later make up for an age-associated waning of other proteostatic pathways, the authors wrote.
What happens to exophers after their expulsion? Twelve hours later, their florescence faded into the surrounding epithelial tissue, suggesting the material was digested. Some protein aggregates did make it through that tissue and into the body cavity, however, and ended up in phagocytic cells there.
Driscoll acknowledges that exophers could be unique to worms. That said, she found tantalizing hints in the literature that point to similar processes taking place in other animals. For instance, the axons of mouse retinal ganglion cells expel worn-out mitochondria for neighboring astrocytes to digest, a process known as transmitophagy (Jun 2014 news).
Mark Ellisman, University of California, San Diego, who co-discovered transmitophagy with Nicholas Marsh-Armstrong of the University of California, Davis, said the two may be related. “I think [exophers] are part of an interesting set of recently observed processes that appear to transfer materials between cells,” he said, adding, “these phenomena call into question how protected one cell is from the materials excreted by other cells in its neighborhood.” Investigating these pathways and their underlying mechanisms more closely may lead to important new understanding of neurodegenerative processes, Ellisman said. Studying how toxic amyloidogenic proteins spread from cell to cell is currently an active area of neurodegeneration research.
Driscoll thinks exophers could help explain how neurodegenerative proteins spread to other cells. Older neurons that produce too few or too many exophers might leave themselves and other neurons vulnerable to toxic proteins. Defining production and clearance pathways could point to whether scientists could ramp up or knock back exophers to treat neurodegeneration, Driscoll said. She plans to manipulate expression of each gene in the C. elegans genome, and also examine neurodegenerative risk variants, to find genes that influence this process.
Other scientists have seen similar structures. “I, and probably others, slapped myself on the head,” said Christopher Link, who studies C. elegans at the University of Colorado at Boulder. “We had seen some of these same things and never put two and two together. I’m completely convinced these findings are real,” Link said. One would think that if an exopher process were afoot in mammalian cells, someone would have seen it, given the size of these sacs; however, much goes unnoticed when you don’t know what to look for. Exophers could have been dismissed as apoptotic bodies or cell fragments broken off by mechanical damage, Link said. People will probably now go review their cultures and images for evidence of this, he added. He is planning to collaborate with Driscoll by sharing data on gene expression changes in Aβ-producing worms to see if those correspond with genes that affect this pathway.
Exophers add to a growing list of tools cells use to rid themselves of waste by passing it off to other cells. Exosomes have been proposed to ferry misfolded proteins away from neurons to their neighbors, as have thread-like structures called tunneling nanotubes that connect distant cells (Dec 2016 news; Gerdes et al., 2013).
Researchers led by Yihong Ye, National Institutes of Health in Bethesda, Maryland, also reported last year on misfolding-associated protein secretion. This MAPS pathway is a newly recognized cellular stress response that releases misfolded proteins into the extracellular space (Jun 2016 news). “Driscoll’s is a very interesting paper with an unexpected finding,” said Ye. He noted that the paper is strong for conducting all of the experiments in vivo, as opposed to in culture, although fluorescently labeled proteins could introduce confounds. Melentijevic said they had checked for that, and found exopher formation in wild-type worms without fluorescent proteins. More mechanistic details about generating and sorting materials into exophers are needed before it can qualify as a bona fide proteostatic mechanism, Ye said.
Chiara Zurzolo, Institut Pasteur, Paris, works on tunneling nanotubes. She sees similarities between those tubes and the threads described in the present paper. Both appear during development and in times of stress and allow passage of mitochondria and lysosomes. However, tunneling nanotubes often pass functional organelles to rescue the receiving cell, not damaged ones that need to be degraded. Nevertheless, Zurzolo said it would be interesting to see if similar pathways are involved in the formation of both types of thread. She agrees that this could be relevant to the prion-like spread of misfolded proteins between cells.—Gwyneth Dickey Zakaib
References
News Citations
- Exosomes: The FedEx of the Nervous System?
- Some Neurons Enlist Glia to Take Care of Their Trash
- Astrocytes and Exosomes Implicated in Protein Propagation
- Can’t Degrade That Pesky Misfolded Protein? Push It Off the MAPS
Paper Citations
- Gerdes HH, Rustom A, Wang X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech Dev. 2013 Jun-Aug;130(6-8):381-7. Epub 2012 Dec 14 PubMed.
Further Reading
Primary Papers
- Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature. 2017 Feb 16;542(7641):367-371. Epub 2017 Feb 8 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.