Shielding Synaptic Glutamate Receptor from Aβ Preserves Memory in Mice
Quick Links
Researchers believe Aβ oligomers do much of their dirty work at the synapse. If so, could protecting synaptic receptors from Aβ preserve cognition? In the May 31 Cell Reports, researchers led by Stephen Ferguson at the University of Ottawa Brain and Mind Institute, Canada, claim as much. The authors chronically administered the small molecule CTEP to two mouse models of AD at the age when memory problems first appear. CTEP binds to mGluR5 (aka metabotropic glutamate receptor 5), preventing Aβ from binding there. After three months, treated mice performed as well as wild-types did in memory tests. Unexpectedly, their amyloid plaque number and soluble aggregated Aβ also fell by half. An analogue of CTEP, basimglurant, has been tested in clinical trials for Fragile X syndrome and major depressive disorder. This compound may be worth evaluating in Alzheimer’s patients as well, Ferguson believes.
“This is an interesting paper that supports the idea that overactivation of mGluR5 by amyloid oligomers, and resulting dysregulated calcium signaling at synaptic locations, play an important role in AD,” Ilya Bezprozvanny at the University of Texas Southwestern Medical Center, Dallas, wrote to Alzforum. Future studies should directly count synapses to confirm they are maintained in treated mice, Bezprozvanny suggested.
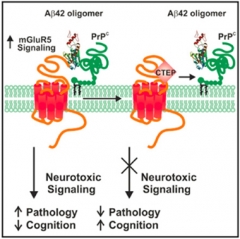
Blocking Synaptotoxicity.
Aβ oligomers and cellular prion protein cooperate to activate toxic signaling through mGluR5 (left), but not when CTEP steps in (right). [Courtesy of Cell Reports, Hamilton et al.]
Previously, several groups reported that Aβ oligomers and cellular prion protein together bind mGluR5, triggering a cascade that releases intracellular calcium and damages synapses (see Jun 2010 news; Sep 2013 news; Haas et al., 2014). These data hinted that blocking mGluR5 could be a therapeutic strategy. To test this, Ferguson and colleagues crossed mGluR5 knockout mice with APPswePS1ΔE9 animals and reported previously that the offspring maintained cognitive skills and, surprisingly, deposited less amyloid than control littermates (see Hamilton et al., 2014). These animals, however, had lacked mGluR5 throughout development. Ferguson wondered if an intervention late in life would have the same effect.
He turned to CTEP, a compound developed by Hoffmann-La Roche, Basel, Switzerland though not for use in people. CTEP penetrates the brain well and selectively targets mGluR5, according to the company (see Lindemann et al., 2011). First author Alison Hamilton administered CTEP to nine-month-old APPswePS1ΔE9 and 3xTg mice every two days for three months. She gave the animals intraperitoneal injections of 2 mg/Kg, a dose that rescued synaptic function and cognition in Fragile X mouse models (see Apr 2012 news). Three months later, treated animals of both strains performed as well as wild-types in object-recognition tests and the Morris water maze. The findings suggest that long-term treatment improved synapse function or maintained synaptic density, Ferguson told Alzforum.
Moreover, treated mice had less amyloid pathology. In both models, the number of plaques in cortex and hippocampus fell by half. In addition, soluble aggregates of Aβ, as measured by an Invitrogen ELISA that does not cross-react with mouse Aβ, fell to non-specific background levels detected in wild-type controls. How mGluR5 inhibition elicits these amyloid changes remains unclear. Activation of the receptor has been found to regulate translation of many mRNA transcripts at the synapse, including amyloid precursor protein, Ferguson noted (see Westmark and Malter, 2007; Apr 2007 news). Thus, antagonizing mGluR5 may directly suppress APP protein levels and Aβ production, an idea Ferguson is now investigating. Other researchers have reported that synaptic activity stimulates endocytosis of APP, leading to more Aβ production, fitting with the idea that blocking mGluR5 might lower Aβ (see Apr 2008 news).
Ferguson is testing how long the beneficial effects of CTEP last, and whether CTEP improves cognition in models of other neurodegenerative diseases such as Huntington’s. He will also explore whether mGluR5 inhibition has negative effects. In this study, wild-type mice chronically given CTEP initially had more trouble with the Morris water maze than controls did. Because mGluR5 plays a crucial role in learning and memory, this could indicate that CTEP treatment harms cognition in normal, healthy brains. In an AD setting, however, the benefits may outweigh ill effects, Ferguson believes.
Roche optimized basimglurant, an allosteric mGlu5 modulator, for use in people. This drug has completed three Phase 2 trials for Fragile X syndrome, but Roche announced the end of this program for lack of efficacy in its Annual Report 2014. The drug also underwent two Phase 2 trials in major depressive disorder (see Lindemann et al., 2015). No further trials are listed on clinicaltrials.gov, however, and as of April 2016, Roche had delisted the compound from its development pipeline. Representatives from Roche declined to comment for this article. An mGluR5 antagonist developed in the 1970s, fenobam, is also in Phase 1 testing for Fragile X syndrome (see Jan 2009 news).—Madolyn Bowman Rogers
References
News Citations
- Aβ Oligomers: A Fatal Attraction for Glutamate Receptors?
- Glutamate Receptor Links Aβ-Prion Complex with Fyn, Synaptic Damage
- Glutamate Receptor Blockers Reverse Fragile X Symptoms in Mice
- Fragile X, MHC Proteins Shape Synapses
- Link Between Synaptic Activity, Aβ Processing Revealed
- mGluR5 Antagonist Crosses First Human Testing Hurdle for Fragile X
Research Models Citations
Paper Citations
- Haas LT, Kostylev MA, Strittmatter SM. Therapeutic molecules and endogenous ligands regulate the interaction between brain cellular prion protein (PrPC) and metabotropic glutamate receptor 5 (mGluR5). J Biol Chem. 2014 Oct 10;289(41):28460-77. Epub 2014 Aug 22 PubMed.
- Hamilton A, Esseltine JL, DeVries RA, Cregan SP, Ferguson SS. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer's disease. Mol Brain. 2014 May 29;7:40. PubMed.
- Lindemann L, Jaeschke G, Michalon A, Vieira E, Honer M, Spooren W, Porter R, Hartung T, Kolczewski S, Büttelmann B, Flament C, Diener C, Fischer C, Gatti S, Prinssen EP, Parrott N, Hoffmann G, Wettstein JG. CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther. 2011 Nov;339(2):474-86. PubMed.
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007 Mar;5(3):e52. PubMed.
- Lindemann L, Porter RH, Scharf SH, Kuennecke B, Bruns A, von Kienlin M, Harrison AC, Paehler A, Funk C, Gloge A, Schneider M, Parrott NJ, Polonchuk L, Niederhauser U, Morairty SR, Kilduff TS, Vieira E, Kolczewski S, Wichmann J, Hartung T, Honer M, Borroni E, Moreau JL, Prinssen E, Spooren W, Wettstein JG, Jaeschke G. Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J Pharmacol Exp Ther. 2015 Apr;353(1):213-33. Epub 2015 Feb 9 PubMed.
External Citations
Further Reading
News
- In APP Knock-Ins, Calcium Chaos Dismantles Mushroom Spines
- Another Side of APP: Does α-Secretase Processing Drive Fragile X Syndrome?
- Immune Receptor Binds Aβ Oligomers, Spurs Synaptic Loss
- Aβ Downs EphB2 Kinase, Disrupts Glutamate Receptors
- Follow the Calcium—Influx Through NMDARs Releases Yet More From ER
- Neuronal Glutamate Fuels Aβ-induced LTD
- Halving Glutamate Receptors Restores Balance in Fragile X Mouse
- Tamping Down Glutamate Receptors Cures Synapses in Fly Retardation Model
Primary Papers
- Hamilton A, Vasefi M, Vander Tuin C, McQuaid RJ, Anisman H, Ferguson SS. Chronic Pharmacological mGluR5 Inhibition Prevents Cognitive Impairment and Reduces Pathogenesis in an Alzheimer Disease Mouse Model. Cell Rep. 2016 May 31;15(9):1859-65. Epub 2016 May 19 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UT Southwestern Medical Center at Dallas
This is an interesting paper by Hamilton and colleagues that supports the idea that overactivation of mGluR5 by amyloid oligomers, and resulting dysregulated calcium signaling at synaptic locations, play an important role in AD. Previous studies already implicated mGluR5 as a target for Aβ oligomers (such as Renner at al., 2010), and Hamilton and colleagues had demonstrated that genetic deletion of mGluR5 alleviates AD phenotypes in mice (Hamilton at al., 2014). Most remarkable in their current study is the observation of beneficial effects in mice treated with CTEP starting at nine months of age. Beneficial effects of CTEP observed in behavioral assays can be potentially explained by synaptoprotective effects. Unfortunately analysis of synaptic loss was not performed by the authors. To explain reduction in amyloid plaques, the authors suggest that the reduction of AD pathology may occur as a consequence of reduced mGluR5-dependent APP translation, but this idea was not tested experimentally. Overall, this study further suggests the importance of synaptic calcium dysregulation in causing AD pathology and implicates mGluR5 as a therapeutic target.
References:
Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010 Jun 10;66(5):739-54. PubMed.
Hamilton A, Esseltine JL, DeVries RA, Cregan SP, Ferguson SS. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer's disease. Mol Brain. 2014 May 29;7:40. PubMed.
Make a Comment
To make a comment you must login or register.