Tau Mediates Neuron Damage in Glaucoma—The Newest Tauopathy?
Quick Links
Glaucoma, the world’s leading cause of blindness, occurs most often in older people. Could it be a form of age-related neurodegeneration? In the May 25 Journal of Neuroscience, researchers led by Adriana Di Polo at the University of Montreal, Canada, propose as much, suggesting that glaucoma may be a tauopathy. In a rat glaucoma model, aggregated, missorted tau accumulated in retinal neurons, the authors found. Conversely, knocking down the protein protected neurons and axons from degeneration. The findings dovetail with reports of amyloid-β plaques in retinas affected by glaucoma, bolstering the idea that it shares features with Alzheimer’s disease. Because the eye is more accessible for research than the brain, it might provide a useful model for studying neurodegeneration, Di Polo believes. “One of the long-term goals of our research is to use the visual system as a springboard to understand more about mechanisms of disease in Alzheimer’s,” she told Alzforum. In particular, she will investigate whether retinal tau buildup could serve as a biomarker of AD.
Commenters found the data intriguing. “This is an elegant and interesting paper,” said Lidia Glodzik at New York University Langone Medical Center. Jochen Herms at the German Center for Neurodegenerative Diseases (DZNE), Munich, called the neuronal protection striking. “[The paper] convincingly indicates that phosphorylated tau is critically involved in neurodegeneration in the retina,” he wrote to Alzforum.
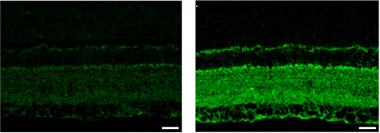
Glaucoma, a Tauopathy?
Tau (green) accumulates in the retina of a rat glaucoma model (right) compared to controls (left). [Courtesy of Chiasseu et al., The Journal of Neuroscience, 2016.]
Previous data had suggested a link between glaucoma and Alzheimer’s. For one thing, glaucoma occurs up to three times more often in AD patients than in the general population (see Bayer et al., 2000). For another, AD patients often suffer from visual problems, such as poor motion perception, and they lose retinal ganglion cells (see Hinton et al., 1986; Blanks et al., 1989). Meanwhile, people with glaucoma have altered levels of Aβ and tau in the eye and cerebrospinal fluid (see Yoneda et al., 2005; Gupta et al., 2008; Nucci et al., 2011). Notably, blocking Aβ protects retinal ganglion cells (RGCs) in glaucoma models (see Aug 2007 news; Salt et al., 2014). Some evidence suggested tau might also damage the retina. In mouse models of tauopathy, RGCs have poor axonal transport and are susceptible to excitotoxicity (see Bull et al., 2012). However, these animals overexpress mutant tau.
Di Polo and colleagues wanted to examine the role of endogenous tau in RGC damage. They turned to a commonly used rat model of glaucoma, in which an injection of hypertonic saline into the eye blocks outflow, jacks up pressure, and triggers loss of RGCs. The authors examined retinas three weeks after injection, when neuronal degeneration had begun. In injured retinas, tau levels were three- to fivefold higher than in controls. Curiously, tau transcripts did not change, suggesting the glut was not due to increased expression. The phosphorylation pattern of tau did change, with some sites more phosphorylated and others less, and these changes correlated with the accumulation of higher molecular weight, oligomeric tau species. Most strikingly, the authors saw a redistribution of tau, with the protein vanishing from the RGC axons of the optic nerve and building up in cell bodies and dendrites, much as has been reported in AD (see Sep 2010 news; Jan 2011 news).
To test tau’s involvement in degeneration, the authors injected short interfering RNA (siRNA) against tau into rat eyes one to two weeks after triggering glaucoma. This suppressed total tau by about 20 percent and oligomeric forms by 75 percent compared to injured, untreated controls. In treated rats, more than 90 percent of RGCs were still alive three weeks after injury, compared to 75 percent in untreated controls. In the optic nerve, 78 percent of axons were intact, contrasting with a roughly 50 percent loss in controls. Pathological tau appears to drive RGC loss in glaucoma, the authors concluded.
Why tau accumulates in RGCs is unclear. Axonal transport falters in RGCs affected by glaucoma, so perhaps this blockade causes the buildup in cell bodies and the dearth of tau in axons, Di Polo suggested (see Salinas-Navarro et al., 2010; Chidlow et al., 2011; Dengler-Crish et al., 2014). Alternatively, tau may aberrantly move out of axons, or tau degradation may slow, she noted. In future work, she will investigate the mechanisms behind the tau rise to determine whether loss from axons or excess in dendrites is more toxic. This information could guide future therapies.
Di Polo believes the data provide a proof of principle that targeting tau could save RGCs. There might be several ways to do this in people. An siRNA strategy against the apoptotic enzyme caspase 2 is being tested in a Phase 1 clinical trial for another eye disease, ischemic optic neuropathy. Other approaches for lowering tau, such as immunotherapy, might also have potential, she suggested.
Glodzik noted that replicating the findings in people will be important, particularly whether reducing intraocular pressure would lower tau levels. Intriguingly, hypertension heightens the risk of developing Alzheimer’s disease, and one study reported a correlation between high blood pressure and increased CSF tau in people who carry the Alzheimer’s risk factor ApoE4 (see Kester et al., 2010). Whether the systemic hypertension effect on tau relates to the elevated intraocular pressure seen in glaucoma remains to be determined, Glodzik noted.
Di Polo plans to study the relationship between Alzheimer’s and glaucoma by inducing the latter in AD mouse models. She wants to develop a retinal imaging probe for tau that could penetrate the cornea. This would allow clinicians to measure retinal tau levels in patients and find out if they rise early in AD or other neurodegenerative diseases. If so, retinal tau imaging might improve diagnosis. Several groups are currently investigating the potential of measuring Aβ in the retina or lens as a diagnostic for AD, but no such test has come near to approval (see May 2013 news; Jul 2014 conference news).—Madolyn Bowman Rogers
References
News Citations
- Glaucoma, AD of the Eye?
- The Plot Thickens: The Complicated Relationship of Tau and Aβ
- Tau’s Synaptic Hats: Regulating Activity, Disrupting Communication
- Not Seeing Eye to Eye: Do Lenses Accumulate Aβ?
- Alzheimer’s Disease: In the Eye of the Patient?
Paper Citations
- Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer's disease. Eur Neurol. 2002;47(3):165-8. PubMed.
- Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986 Aug 21;315(8):485-7. PubMed.
- Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer's disease. Brain Res. 1989 Nov 6;501(2):364-72. PubMed.
- Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005 Mar-Apr;49(2):106-8. PubMed.
- Gupta N, Fong J, Ang LC, Yücel YH. Retinal tau pathology in human glaucomas. Can J Ophthalmol. 2008 Feb;43(1):53-60. PubMed.
- Nucci C, Martucci A, Martorana A, Sancesario GM, Cerulli L. Glaucoma progression associated with altered cerebral spinal fluid levels of amyloid beta and tau proteins. Clin Experiment Ophthalmol. 2011 Apr;39(3):279-81. PubMed.
- Salt TE, Nizari S, Cordeiro MF, Russ H, Danysz W. Effect of the Aβ aggregation modulator MRZ-99030 on retinal damage in an animal model of glaucoma. Neurotox Res. 2014 Nov;26(4):440-6. Epub 2014 Aug 9 PubMed.
- Bull ND, Guidi A, Goedert M, Martin KR, Spillantini MG. Reduced axonal transport and increased excitotoxic retinal ganglion cell degeneration in mice transgenic for human mutant P301S tau. PLoS One. 2012;7(4):e34724. Epub 2012 Apr 4 PubMed.
- Salinas-Navarro M, Alarcón-Martínez L, Valiente-Soriano FJ, Jiménez-López M, Mayor-Torroglosa S, Avilés-Trigueros M, Villegas-Pérez MP, Vidal-Sanz M. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Exp Eye Res. 2010 Jan;90(1):168-83. Epub 2009 Oct 14 PubMed.
- Chidlow G, Ebneter A, Wood JP, Casson RJ. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011 Jun;121(6):737-51. Epub 2011 Feb 11 PubMed.
- Kester MI, van der Flier WM, Mandic G, Blankenstein MA, Scheltens P, Muller M. Joint effect of hypertension and APOE genotype on CSF biomarkers for Alzheimer's disease. J Alzheimers Dis. 2010;20(4):1083-90. PubMed.
External Citations
Further Reading
Primary Papers
- Chiasseu M, Cueva Vargas JL, Destroismaisons L, Vande Velde C, Leclerc N, Di Polo A. Tau Accumulation, Altered Phosphorylation, and Missorting Promote Neurodegeneration in Glaucoma. J Neurosci. 2016 May 25;36(21):5785-98. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Ludwig-Maximilians-Universität Munich
This is a nice paper on retinal pathology in glaucoma that convincingly indicates that phosphorylated tau is critically involved in neurodegeneration in the retina. Targeted tau siRNA led to striking protection of ocular hypertension-induced neuronal damage. This finding does contradict somewhat our study in a transgenic tau mouse model. We did not see a loss of RGCs that accumulated aggregated tau in high amounts (Schön et al., 2012). But analyzing the loss of endogenous tau, as in the present study, is a very different approach from examining the overexpression of mutant human tau that forms fibrillary tau aggregates in mouse RGCs.
References:
Schön C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, Seeliger MW, Arzberger T, Goedert M, Kretzschmar HA, Schmidt B, Herms J. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7(12):e53547. PubMed.
Make a Comment
To make a comment you must login or register.