ALS Gene Tied to Psychiatric and Neurodevelopmental Disease
Quick Links
Mutations in the C9ORF72 gene cause both the fatal motor-neuron disease amyotrophic lateral sclerosis and frontotemporal dementia, the second-leading cause of early onset dementia after Alzheimer’s. The gene may bear other bad news, according to Emma Devenney, John Hodges, and colleagues at the University of Sydney. They report that relatives of people with ALS or FTD due to the hexanucleotide expansion of C9ORF72 have more schizophrenia, psychosis, suicide, and autism-spectrum disorders than do families with ALS/FTD stemming from other genetic causes. Their study builds the case that these two neurodegenerative diseases share genetic links with psychiatric and developmental disorders, and it nominates C9ORF72 as an important source of the shared risk. The findings were published September 26 in Neurology.
- Relatives of people with ALS or FTD are at higher risk for mental illness.
- They are more likely to have psychosis, schizophrenia, or autism.
- C9ORF72 expansions are behind some of this risk.
Links between ALS and schizophrenia were apparent early on. Original descriptions of ALS from the 1940s note frequent psychotic features. More recently, researchers reported higher rates of schizophrenia, suicide, obsessive-compulsive disorder, autism, and alcoholism in families of Irish ALS patients than among families of age-matched controls (Oct 2017 news; Byrne et al., 2013). Others found direct evidence that a portion of the genetic liability for ALS and schizophrenia is shared (McLaughlin et al., 2017). FTD and schizophrenia also cluster in families (Schoder et al., 2010).
To find out if C9ORF72 is responsible for some of this overlap, Devenney collected detailed family histories on 46 patients with behavioral variant FTD (bvFTD) and 43 with ALS. Of the 89, 29 had the C9ORF72 expansion. Devenney asked about diagnoses of depression, anxiety, bipolar disorder, schizophrenia, psychosis, and suicide, as well as ALS and FTD in a total of 1,414 of their first- and second-degree relatives.
C9ORF72 did make a difference. Among family members of C9ORF72 carriers, 14 percent had been diagnosed with a mental illness, compared with 7 percent of family members of noncarriers. The frequency of mental illness did not differ between families with ALS or FTD, suggesting that the increased incidence is linked to the genetic defect, not to either clinical diagnosis.
Relatives of C9ORF72 carriers among the 89 patients were more likely to have mental illness diagnoses than relatives of noncarriers. Odds of being diagnosed with psychosis, schizophrenia, suicide, or autism spectrum disorder (ASD) were 19.9, 4.9, 2.7, and 2.7 times higher, respectively, than the odds among noncarrier relatives. The risk of bipolar disorder was the same for relatives of carriers and noncarriers. The psychiatric conditions were equally represented in the ALS and FTD families, while autism-spectrum disorders were slightly more common in FTD families.
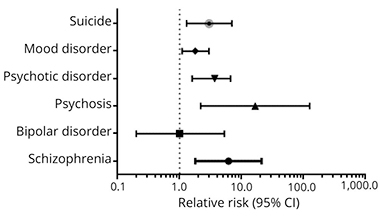
Pernicious Pedigree.
Having a relative with ALS or FTD due to C9ORF72 raises a person’s odds of schizophrenia, psychosis, and suicide, but not bipolar disorder, compared with families with ALS or FTD due to other causes. [Republished with permission. © 2018 American Academy of Neurology.]
Because the relatives were not genotyped, the authors do not know if C9ORF72 expansions associated with their psychiatric conditions. Devenney said they hope to answer this question with more data from their ongoing studies of presymptomatic carriers, which will genotype and follow family members for signs of diseases.
How could the C9ORF72 expansion lead to such a wide variety of clinical outcomes? Devenney does not know, but pointed out that ALS, FTD, and the other diseases share symptoms, such as psychosis and changes in language and behavior. FTD and autism both produce ritualistic and compulsive behaviors; apathy is a feature of both schizophrenia and FTD. “A lot of those behaviors seem to map onto abnormalities in specific brain networks, which may be a point of vulnerability in these families. But why some get disease in childhood, or teenage years, and others at the end of the life, we don’t know. There must be some kind of genetic or epigenetic factors, but that’s not clear just yet,” she said.
Devenney, a neurologist, hopes her data will help bridge traditionally distinct fields. “The results open up the possibility of collaborating across neurology and psychiatry, which I think is exciting. This will give us new avenues for research, and possibly new treatments down the line,” she said.
The study is remarkable and credible, wrote Vishwajit Nimgaonkar, University of Pittsburgh. His group has studied co-segregation of schizophrenia with other diseases in families. Nimgaonkar found a handful of schizophrenia patients with heritable C9ORF72 expansion (Watson et al., 2016), but no history suggestive of FTD/ALS. Nonetheless, he’s interested in the idea that subtle neurodegenerative processes can occur in schizophrenia.—Pat McCaffrey
References
News Citations
Paper Citations
- Byrne S, Heverin M, Elamin M, Bede P, Lynch C, Kenna K, Maclaughlin R, Walsh C, Al Chalabi A, Hardiman O. Aggregation of neurologic and neuropsychiatric disease in amyotrophic lateral sclerosis kindreds: A Population-Based Case-Control Cohort Study of Familial and Sporadic Amyotrophic Lateral Sclerosis. Ann Neurol. 2013 Jul 9; PubMed.
- McLaughlin RL, Schijven D, van Rheenen W, van Eijk KR, O'Brien M, Kahn RS, Ophoff RA, Goris A, Bradley DG, Al-Chalabi A, van den Berg LH, Luykx JJ, Hardiman O, Veldink JH, Project MinE GWAS Consortium, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genetic correlation between amyotrophic lateral sclerosis and schizophrenia. Nat Commun. 2017 Mar 21;8:14774. PubMed.
- Schoder D, Hannequin D, Martinaud O, Opolczynski G, Guyant-Maréchal L, Le Ber I, Campion D. Morbid risk for schizophrenia in first-degree relatives of people with frontotemporal dementia. Br J Psychiatry. 2010 Jul;197(1):28-35. PubMed.
- Watson A, Pribadi M, Chowdari K, Clifton S, Joel Wood, Miller BL, Coppola G, Nimgaonkar V. C9orf72 repeat expansions that cause frontotemporal dementia are detectable among patients with psychosis. Psychiatry Res. 2016 Jan 30;235:200-2. Epub 2015 Dec 8 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Devenney EM, Ahmed RM, Halliday G, Piguet O, Kiernan MC, Hodges JR. Psychiatric disorders in C9orf72 kindreds: Study of 1,414 family members. Neurology. 2018 Oct 16;91(16):e1498-e1507. Epub 2018 Sep 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Pittsburgh School of Medicine
I think this study is remarkable and credible. Our group has been interested in co-segregation of schizophrenia and other diseases in families. We surveyed DNA from our stored samples collected from patients with schizophrenia and found a handful with heritable C9ORF72 expansions (Watson et al., 2016). Interestingly, those patients had florid psychoses but there was no history suggestive of FTD/ALS. Over 20 years ago, several groups had reported increased prevalence of tri-nucleotide repeat expansions in schizophrenia, fueling speculation about the hypothesis that subtle neurodegenerative processes can occur in schizophrenia. I am very interested in collaborating with FTD/ALS researchers interested in this phenomena.
References:
Watson A, Pribadi M, Chowdari K, Clifton S, Joel Wood, Miller BL, Coppola G, Nimgaonkar V. C9orf72 repeat expansions that cause frontotemporal dementia are detectable among patients with psychosis. Psychiatry Res. 2016 Jan 30;235:200-2. Epub 2015 Dec 8 PubMed.
University of Pennsylvania
We noted in a study by Geser et al., 2010, that pathological 43-kDa transactivation response DNA-binding protein (TDP-43) was present to a variable extent in the postmortem brains of 29 percent of longitudinally followed older (>age 65) schizophrenia patients, but also in 29 percent older adults without severe mental illness over age 65. The similar findings of TDP-43 pathology in elderly patients with severe mental illness and controls suggest common age-dependent TDP-43 changes in limbic brain areas. These data provide an age-related baseline for the development of whole-brain pathological TDP-43 evolution schemata.
References:
Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 2010 Oct;67(10):1238-50. PubMed.
Make a Comment
To make a comment you must login or register.