NIH Summit Examines What Makes a Healthy Aging Brain
Quick Links
Neurodegenerative disease researchers spend a lot of time considering what goes wrong in the brain. But what about when things go right? Factors that contribute to healthy brain aging took center stage at the Cognitive Aging Summit held April 6 to 7 at the Bethesda North Marriott Hotel & Conference Center in Maryland. More than 300 researchers—some working on human cohorts, other animal models, even gaming technology—gathered to share data. They focused on the concepts of cognitive resilience and reserve in exploring exactly what happens when the brain ages successfully. Co-organized by Molly Wagster and Jonathan King at the National Institute on Aging (NIA), Bethesda, Maryland, this summit was the third since 2007. It aimed to collate the field’s knowledge and hammer out research priorities for the future.

“This is an uncommon opportunity to get scientists from all these areas of expertise to converge,” NIA director Richard Hodes told Alzforum. “The relevance and implications of one line of research for another becomes clearer in this setting.”
Yaakov Stern, Columbia University, New York, agreed. “Some scientists have human cohorts, autopsied brains, or epidemiological studies, and others use animal models,” he told Alzforum. “There are many approaches and thought processes brought to bear on how to best understand the phenomenon of reserve.”
NIA and the McKnight Brain Research Foundation (MBRF) in Orlando, Florida, established a Research Partnership in Cognitive Aging in 2006. Administered by the Foundation for the National Institutes of Health (FNIH), its goal was to stimulate progress on brain aging through research funding. The partnership sponsored the first cognitive aging summit, identified priority areas for future study, and led to MBRF/NIA funding of 17 research grants to the tune of $28 million. The project included neural and behavioral changes during aging, and interventions to prevent or treat impairment. A 2010 summit spawned a $15 million grant for a multicenter trial testing whether stress reduction and exercise can affect age-related cognitive decline.
Despite these efforts, no concrete measures have emerged to support an aging brain. The NIA commissioned the Agency for Healthcare Research and Quality in Rockville, Maryland, to conduct a literature review. It concluded in March that there was insufficient evidence that any intervention to date—supplements, medications, lifestyle changes, or cognitive training—delays or prevents age-related cognitive decline, mild cognitive impairment, or AD.
At this year’s summit, leaders in the field focused on cognitive reserve. The idea is that cognitive reserve helps the brain preserve cognition in the face of ongoing pathology, and if scientists better understand the processes involved, maybe they can boost reserve. Conference presentations ran the gamut of challenges in this field, from how to conceptualize reserve and measure it, to how to mine animal and human data. In addition, researchers discussed possible opportunities to remediate aging.
Can We Sharpen A Hazy Definition?
Scientists generally accept that education and intellectual enrichment contribute to cognitive reserve, and that people with a lot of it live dementia-free longer (see Jun 2014 news). Beyond that, the concept remains something of a black box. Most researchers at the summit agreed that the definition originally proposed by Stern represents a starting point, but needs to evolve to more concrete terms (see Stern, 2012). Stern said that reserve modulates the effect of injury on cognition, such that for a given degree of pathology, people with more reserve show less cognitive decline. He agreed that a mechanistic definition has yet to emerge. “We have a long way to go before everybody’s on the same page,” he told Alzforum. Similarly, scientists debated how to define related terms such as “resilience” and “compensation,” often using them interchangeably.
Claudia Kawas, University of California, Irvine, noted that “solid definitions would help streamline the field’s thinking and shape future research studies,” much as consensus definitions of Alzheimer’s disease, mild cognitive impairment, and preclinical AD helped advance the field.
Clues from Animals
Against a nebulous backdrop, scientists have turned to animal models of aging, studying mice and rats that stay cognitively unchanged as they age, in contrast to littermates whose ability to remember environments or familiar objects declines. Since wild-type animals age without accumulating the same brain pathologies as humans, researchers can observe “pure” aging, they said.
For instance, Michela Gallagher, Johns Hopkins University, Baltimore, studies brain aging in rats. She has observed that older rats that gradually lose their cognition have overactive hippocampi, indeed hyperactivity in regions analogous to the default mode network (DMN) in humans. Microarray profiling and electrophysiological recordings suggest that aged cohorts with preserved memory function correct the overexcitation by ramping up inhibition, Gallagher said. Since she and other groups have observed hippocampal hyperactivity in older people with memory impairment as well, this suggests common signals of normal aging across species, she said.
Similarly, Sara Burke, University of Florida, Gainesville, reported differences in neuronal activity between rats that aged well and rats that did not. She performed gene expression analyses of immediate early genes just after rats trained on a complex memory task that depended on connections between the hippocampus, perirhinal cortex, and prefrontal cortex. Her data suggested the perirhinal cortices were less active in old than in young rats. At the same time, old rats with superior performance ramped up prefrontal cortical firing. This resembles the rise in prefrontal cortical activity that has been observed in high-performing older people, she said (Davis et al., 2008). “It suggests the prefrontal cortex may try to compensate as the perirhinal cortex becomes dysfunctional,” she said.
Other researchers are using animals to try to figure out what goes on at the molecular level as the brain ages. Marcelo Wood, University of California, Irvine, studies the role of the histone deacetylase HDAC3, which is expressed predominantly in the brain and represses gene expression. His group knocked out the HDAC3 gene in the dorsal hippocampi of mice, then trained them at young or old ages on a novel object-location task. Young mice performed equally well, regardless of whether they expressed HDAC3. In older animals the story was different. Wild-type animals became forgetful, whereas HDAC3-deficient mice remembered just as well as did young mice. This suggests HDAC3 hampers memory as mice age, Wood told the audience. In keeping with this, long-term potentiation weakened with age in wild-type but not HDAC3 knockout mice. Since knocking out HDAC3 restored hippocampal expression of Period1 (Per1), a master regulator of the cellular circadian clock, HDAC3 might function to help regulate circadian genes in the hippocampus, Wood said.
Others suspect inflammatory processes. Researchers led by Thomas Foster, also from UF Gainesville, are studying how inflammatory cytokines influence brain activity. Many of these are increasingly released by immune cells as rats age. Foster shared preliminary 4.7T functional MRI data in rats, hinting that when researchers genetically overexpressed interleukin 6 levels in the blood, the correlation of activity between the ventral hippocampus and prefrontal cortex weakened. A similar signal in aging humans has raised the possibility that the reduced brain connectivity might be related to inflammation, Foster said.
Aging Well Despite Pathology and Genetic Risk
What can researchers glean from studying people, notably the lucky few who make it into their 80s and 90s with crystal-clear memories? Some of these spry minds have advanced pathology in their brains, while others harbor genetic risk factors for cognitive decline.
Data from the 90-plus study, led by Kawas, suggest that at very old ages, the amount of amyloid in the brain has little bearing on how fast people decline cognitively in the years before death. In fact, the eight highest performers on memory tests who have come to autopsy had a wide range of pathology, ranging from little to full-blown plaques and tangles. This adds to evidence that a low pathology burden doesn’t explain better memory in older people, Kawas concluded. In support of that idea, Patricia Boyle, Rush University Medical Center, Chicago, analyzed 1,200 autopsied brains from the Rush Memory and Aging Project and found brain pathology explained only 40 percent of cognitive decline. That leaves 60 percent of total impairment unexplained, she said. “Neuropathologies are important, but they are not the whole story.”
To find out what could be protecting brains in the face of protein aggregation, Susan Resnick at the NIA plans to examine how more than 350 ApoE4 carriers in the Women's Health Initiative Memory Study have made it to age 80 with no cognitive impairment. Tammie Benzinger of the Washington University School of Medicine in St. Louis plans a substudy of familial AD mutation carriers in the Dominantly Inherited Alzheimer’s Network (DIAN) who are well past their expected age of onset, have loads of plaques and tangles in their brains, and yet have normal structural MRI scans and no symptoms of AD.
One possibility comes from the lab of Emily Rogalski, Northwestern University, Chicago. Her data suggest that atrophy, or the lack thereof, may play a role in resilience to age-related memory loss. She reported in the April 4 JAMA that the cortices in a cohort of 24 superagers—people over 80 with episodic memory scores typical of middle age—shrank at half the rate of the average 80-year-old (see Cook et al., 2017).
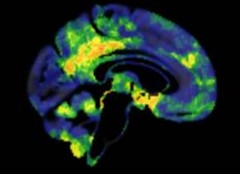
Bulked Up.
Cognitively normal older people at heightened risk for Alzheimer’s may be temporarily protected by more brain volume (orange/yellow) in medial brain areas compared to low-risk controls. [Courtesy of William Jagust.]
Seconding the notion that higher brain volume is protective, William Jagust, University of California, Berkeley, and postdoctoral collaborator Eider Arenaza-Urquijo from Gaël Chételat’s lab at INSERM, Paris, looked at amyloid-positive, cognitively normal people over age 70. The researchers compared 23 participants with an APOE4 allele or a family history of Alzheimer’s to age- and education-matched controls. In the high-risk group, medial temporal, prefrontal, and parietal areas had more volume, while the prefrontal cortex metabolized more glucose (see image). Since this group was cognitively normal, and performed better on average on a test of episodic memory than the low-risk controls, these factors seem to be protective. However, Jagust pointed out that over subsequent assessments, this high-risk group declined faster, suggesting the protection only lasts so long.
How to Slow, or Even Turn Back the Clock?
Gareth Howell of the Jackson Laboratory in Bar Harbor, Maine, presented mouse data suggesting a healthy diet improved myelination and white-matter integrity. Exercise did, too, even when the mice ate a diet high in fat and sugar. Ozioma Okonkwo, University of Wisconsin, Madison, reported that exercise abolished the effects of age on a number of AD-related outcome measures in middle-aged adults from the Wisconsin Registry for Alzheimer’s Prevention. Whereas sedentary people accumulated amyloid, metabolized less glucose, had smaller hippocampi and worse episodic memory with aging, participants who exercised had minimal age-related changes on these markers. In other words, exercise kept brains young. In response to an audience question, Okonkwo agreed that the effect could go through better vascular health.
Other researchers talked about how challenging it is to validate high-tech methods under investigation. Joel Voss, Northwestern University, Evanston, Illinois, is testing the effects of transcranial magnetic stimulation on the neural networks of the hippocampus in people. He emphasized that scientists need to take special care to validate that TMS is useful before moving forward. “The methods we are using are complex, hard to control, and the opportunity for placebo and nonspecific effects is huge,” he said. To guard against those dangers, Voss stressed the need to rigorously design experiments and statistical analyses. It’s also crucial to prove that method has in fact engaged the intended brain target, he said.
Those challenges extend to virtual reality and video games as well. Scientists led by Adam Gazzaley, University of California, San Francisco, have been creating games to improve cognition. How can they tell if those games have lasting benefits over time that generalize to daily activities? One method they are trying uses a digital “glass brain” model, created for each person. It uses a person’s MRI data to construct a three-dimensional representation of his or her cortical and white matter, then overlays that person’s real-time wireless EEG data, which is recorded as the person plays a game with electrodes glued to his or her scalp. During play, the model helps monitor how the player’s brain responds to the cognitive stimuli and whether and how that response changes over time (see image below).

Glass Brain: A three-dimensional model melds the cortical surface, white matter (gold), and EEG data (green dots and dashes) of a person to track how the brain responds in real time as he or she plays a video game. [Courtesy of Adam Gazzaley.]
But that’s not enough for validation, Gazzaley said. He described efforts to construct double-blind trials using “placebo” games. These games are designed to seem beneficial to participants, without actually exercising the cognitive domains being tested. Researchers monitoring the EEG data are also blinded to which game a person is playing. That way, both participants and researchers stay in the dark about whether their game is the “real” one. Recent reports have questioned the real-world benefit of video games purported to improve cognition (see Jan 2016 news).
After the conference, MBRF trustees, NIA leaders, and session chairs met behind closed doors to set recommendations for future research priorities. Conference proceedings will appear as six published papers in the journal Neurobiology of Aging in 2018, Wagster said, noting that videos of the sessions will be available in May.—Gwyneth Dickey Zakaib
References
News Citations
- A Life of Cognitive Enrichment May Fend Off Dementia. But How?
- Game Over? Federal Trade Commission Calls Brain-Training Claims Inflated
Paper Citations
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012 Nov;11(11):1006-12. PubMed.
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008 May;18(5):1201-9. Epub 2007 Oct 8 PubMed.
- Cook AH, Sridhar J, Ohm D, Rademaker A, Mesulam MM, Weintraub S, Rogalski E. Rates of Cortical Atrophy in Adults 80 Years and Older With Superior vs Average Episodic Memory. JAMA. 2017 Apr 4;317(13):1373-1375. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.