Tau Receptor Identified on Cell Surface
Quick Links
Evidence now seems overwhelming that toxic forms of tau spread from cell to cell, causing the characteristic progression of tauopathies such as Alzheimer’s and frontotemporal dementia. But how does tau get from one cell to another? At Tau2020, a new conference held February 12 and13 in Washington, D.C., Ken Kosik, University of California, Santa Barbara, reported that tau slips into cultured human neurons by binding LDL-receptor-related protein 1 (LRP1), a cell surface receptor. In mice, knocking down LRP1 expression shut down the spread of injected mutant tau. “LRP1 is a master regulator of tau uptake and spread,” Kosik told the audience.
- Tau binds LDL-receptor-related protein 1.
- Neurons lacking this receptor do not endocytose tau.
- Suppressing LRP1 stops tau transmission in mice.
LRP1 popped up as a hit when Jennifer Rauch, a postdoc in Kosik’s lab, used a CRISPR-based screen to search for receptors of various forms of tau, including oligomers and fibrils.
Previously, Rauch had developed a cell-based assay for tau uptake based on a fluorescently labeled tau reporter. In essence, she added labeled tau into cell culture medium, then used flow cytometry to measure its incorporation into the cells over time. This confirmed what others had reported, namely that heparin sulfate proteoglycans (HSPGs) seem to mediate tau uptake (Rauch et al., 2018; Apr 2015 news). However, even after blocking HSPGs, some tau still entered cells, suggesting there are other receptors.
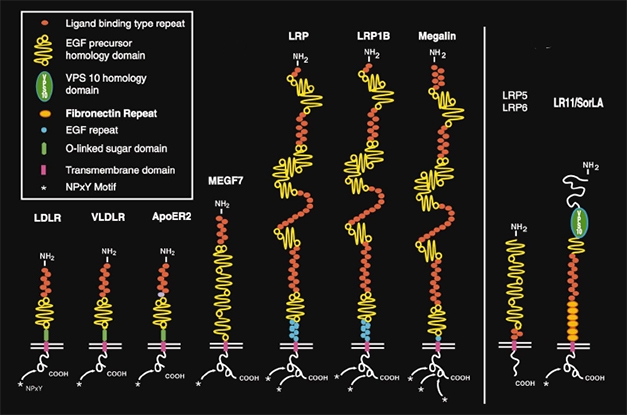
LDLR Family. LRP1 belongs to the LDL receptor family (left). These, and more distantly related cell surface receptors (right), bind a wide range of proteins and play important roles in cholesterol metabolism, endocytosis, and cell signaling in the brain. [Courtesy Joachim Herz, University of Texas Southwestern Medical Center, Dallas, and Neuron.]
To cast a wider net, Rauch adopted a candidate approach. She used the same assay with cells she had denuded of various cell-surface receptors. Specific guide RNAs and CRISPR ensured that H4 neuroglioma cells lacked various members of the LDL receptor family.
Rauch found that knockdown of LRP1 totally abolished tau uptake. In contrast, ridding the cells of LRP1 homologs, including LDLR, LRP1B, LRP2, LRP5, LRP8, and VLDLR, had no effect. Without LRP1, the H4 cells were unable to absorb monomers, oligomers, and fibrils of tau. Likewise, when Rauch knocked down LRP1 in neurons derived from human induced pluripotent stem cells, they also no longer endocytosed tau. “The results were dramatic,” said Kosik.
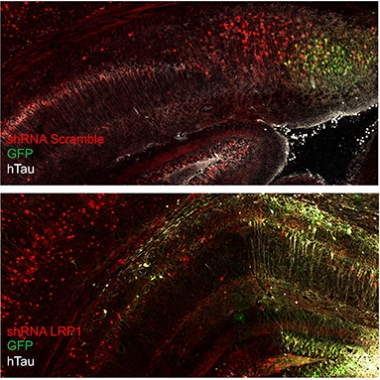
Don’t Spread ’Em. In the mouse brain, human mutant tau (white) can spread from donor cells (green) to recipient cells. Knocking down LRP1 (bottom) arrests the spread, restricting human tau to donor cells. [Courtesy Jenny Rauch, UCSB.]
Cultured cells are well and good, but does LRP1 mediate cell-to-cell transmission of tau in the brain? To find out, Rauch used a mouse model developed by Susanne Wegmann. When at Brad Hyman’s lab at Massachusetts General Hospital, Charlestown, Wegmann wanted to distinguish tau made in cells from tau taken up by cells. She engineered an adeno-associated virus encoding a chimera of enhanced green fluorescent protein coupled via a 2A peptide domain to human P301L mutant tau. The linker undergoes self-proteolysis, releasing tau from eGFP once the chimera is expressed. Hence, tau “donor” cells show up by their GFP fluorescence, and “recipient” cells are marked by tau immunohistochemistry but no GFP fluorescence (Wegmann et al., 2019).
Rauch used this AAV reporter to track the spread of tau. First, though, she injected, retro-orbitally, 6-week-old wild-type mice with a short-hairpin RNA to knock down expression of LRP1 in their brains. Retro-orbital injections into the sinuses is commonly used instead of tail vein injections for systemic delivery of a variety of agents. Two weeks later, she injected the AAV, intracerebroventricularly, into the brain. Three weeks after that, she killed the mice and looked for human tau throughout their brains. In mice injected with a scrambled shRNA and normal LRP1expression, human P301L tau lit up all through the hippocampus and connected cortical areas. In mice treated with the LRP1 shRNA, human tau was restricted to a tiny focus of eGFP fluorescence at the AAV injection site. In short, knocking down LRP1 had prevented the spread of the mutant tau.
Kosik does not know what type of brain cells tau entered. The cells bound MC1, an antibody that recognizes a conformation of tau found in neurofibrillary tangles.
How does LRP1 bind tau? The receptor is one of the largest in the LDLR family, having four cysteine-rich ligand binding domains. It is well-known and promiscuous, binding more than 40 different ligands, including α2 macroglobulin, amyloid precursor protein, Aβ, and ApoE (Holtzman et al., 1995; Fuentealba et al., 2010). It wears many hats, being important for cholesterol homeostasis, endocytosis, and cell signaling through its many ligands.
To figure out which LRP1 domain might hook tau, Rauch made mini receptors, each having only one of its normally four binding domains. Cells expressing ligand binding domain IV had highest affinity for tau. Domain IV binds receptor-associated protein (RAP), a common ligand for most LDLR family members. Looking at specific tau regions, Rauch found that tau’s microtubule binding domains bound to LRP binding domain IV, while tau’s N-terminal bound to ligand binding domain II.
Crystal structures of RAP bound to LRP1 have been solved, noted Kosik, and they are guiding scientists with regard to how tau binds LRP1. For example, lysines on RAP bind to aspartic acid residues on LRP1 through salt bridges. Tau may bind similarly, since capping tau lysines with a chemical called sulfo-NHS-acetate completely abolished endocytosis via LRP1.
Researchers at the Tau2020 meeting were excited by the findings. They asked how LRP1 fits with HSPG’s role in tauopathies. Might both bind tau simultaneously, either in a ternary complex, or even a quaternary one with ApoE? Kosik said this remains to be sorted out. He speculated that HSPGs act as catch-alls, scooping various molecules out of the extracellular space and bringing them closer to the cell surface, where more specific interactions take place.
As for ApoE, it, like RAP, seems to suppress endocytosis of tau, at least in cell assays. All isoforms of ApoE behave similarly in this regard. Kosik noted that LRP1 binds ApoE in a region where the protective Christchurch mutation is located (Nov 2019 news). “It would be worth looking into how this mutation might affect ApoE4-LRP binding and tau dynamics,” said Kosik.—Tom Fagan
References
News Citations
Antibody Citations
Paper Citations
- Rauch JN, Chen JJ, Sorum AW, Miller GM, Sharf T, See SK, Hsieh-Wilson LC, Kampmann M, Kosik KS. Tau Internalization is Regulated by 6-O Sulfation on Heparan Sulfate Proteoglycans (HSPGs). Sci Rep. 2018 Apr 23;8(1):6382. PubMed.
- Wegmann S, Bennett RE, Delorme L, Robbins AB, Hu M, McKenzie D, Kirk MJ, Schiantarelli J, Tunio N, Amaral AC, Fan Z, Nicholls S, Hudry E, Hyman BT. Experimental evidence for the age dependence of tau protein spread in the brain. Sci Adv. 2019 Jun;5(6):eaaw6404. Epub 2019 Jun 26 PubMed.
- Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9480-4. PubMed.
- Fuentealba RA, Liu Q, Zhang J, Kanekiyo T, Hu X, Lee JM, LaDu MJ, Bu G. Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One. 2010;5(7):e11884. PubMed.
Further Reading
Papers
- Shinohara M, Tachibana M, Kanekiyo T, Bu G. Role of LRP1 in the pathogenesis of Alzheimer's disease: evidence from clinical and preclinical studies. J Lipid Res. 2017 Jul;58(7):1267-1281. Epub 2017 Apr 4 PubMed.
- Eggert S, Thomas C, Kins S, Hermey G. Trafficking in Alzheimer's Disease: Modulation of APP Transport and Processing by the Transmembrane Proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin. Mol Neurobiol. 2017 Oct 27; PubMed.
- Storck SE, Pietrzik CU. Endothelial LRP1 - A Potential Target for the Treatment of Alzheimer's Disease : Theme: Drug Discovery, Development and Delivery in Alzheimer's Disease Guest Editor: Davide Brambilla. Pharm Res. 2017 Dec;34(12):2637-2651. Epub 2017 Sep 25 PubMed.
- Itakura E, Chiba M, Murata T, Matsuura A. Heparan sulfate is a clearance receptor for aberrant extracellular proteins. J Cell Biol. 2020 Mar 2;219(3) PubMed.
Primary Papers
- Rauch JN, Luna G, Guzman E, Audouard M, Challis C, Sibih YE, Leshuk C, Hernandez I, Wegmann S, Hyman BT, Gradinaru V, Kampmann M, Kosik KS. LRP1 is a master regulator of tau uptake and spread. Nature. 2020 Apr;580(7803):381-385. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.