Umibecestat-Driven Cognitive Decline Is Reversible
Quick Links
At this year’s virtual Alzheimer’s Association International Conference, researchers from Novartis and the Banner Alzheimer’s Institute presented highly anticipated follow-up data from the massive Generation 1 and 2 Phase 3 trials. These were halted when volunteers on the BACE inhibitor umibecestat began to develop subtle memory problems. Since then, volunteers and researchers both have been asking if that decline would be permanent. At AAIC, API investigators presented hot-off-the-press data to indicate that no, it wasn’t.
- In Generation trials, umibecestat caused memory loss.
- Memory recovered after volunteers stopped taking the drug.
- Subtle brain atrophy also resolved.
The trials, supported by Amgen and the NIH, tested whether umibecestat would prevent Alzheimer’s dementia in healthy carriers of the ApoE4 allele. One ApoE4 copy increases a person’s odds of getting the disease three- to fourfold; two copies, up to 15-fold. Dosing ended abruptly last summer when a preplanned interim analysis detected worsening cognition in people taking the BACE inhibitor (July 2019 news).
In her AAIC presentation, Ana Graf from Novartis in Basel, Switzerland, showed that several months after participants had taken their last dose, no differences remained between active and placebo groups. Eric Reiman from Banner Alzheimer’s Institute in Phoenix reported the same for brain volume lost during the trial.
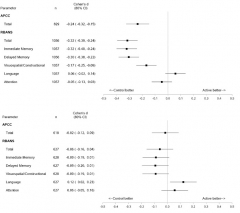
Cognition Restored. While taking umibecestat, volunteers performed worse on cognitive tests than did those on placebo, as seen in their last assessment on drug (top). Four months after they stopped, differences between the groups had all but disappeared (bottom). [Image courtesy API.]
Graf summed up data from the API Preclinical Composite Cognitive test and the RBANS. The APCC, designed to detect subtle cognitive change in healthy volunteers, was a primary outcome of the Generation trials. The RBANS, a commercial battery comprising immediate memory, delayed memory, attention, language, and visuospatial/constructional indices, was a secondary outcome; it was also used to assess safety during the trials. The RBANS was the principal measure that lead to the discontinuation.
To learn if the side effects were permanent, Graf and colleagues looked to volunteers who had taken 15 mg or 50 mg umibecestat daily for at least two months. These 1,056 participants had slipped on both the RBANS and the APCC, performing worse than those on placebo. Cohen’s d values of about 0.25 and -0.32 for the APCC and RBANS, respectively, indicated small to medium effect sizes. Poor immediate and delayed recall drove the RBANS deficit.
Graf noted that the difference between the drug and placebo groups was not wholly due to worsening on drug, but also to a greater proportion of people in the placebo group than on drug improving on the tests. Practice effects often lead to better cognitive scores as trials in asymptomatic or very mildly symptomatic participants progress. Practice effects have been a problem in the DIAN trials of solanezumab and gantenerumab, as well (Apr 2020 conference news).
What happened after volunteers stopped umibecestat? A median of four months later, the difference between placebo and drug groups had all but vanished, with Cohen’s d values of -0.02 for the APCC and -0.06 for the RBANS. The reversal occurred across all subgroups, namely people who were amyloid-positive or -negative at baseline and in both ApoE4 homozygotes and heterozygotes. The amyloid-positive homozygotes had declined most on drug, with Cohen’s d values of -0.55 and -0.42 for the APCC and RBANS, respectively, but following washout these differences had fallen to +0.01 for the APCC, and -0.12 for the RBANS. Graf concluded that there was no long-term impact on disease progression.
Reiman reached a similar conclusion based on MRI analysis. Pooled data from both trials indicated more loss of hippocampal and whole-brain volume in treatment groups by week 26, irrespective of dose. Cohen’s d values of -0.45 and -0.31 for whole brain and hippocampus, respectively, indicated medium effect sizes. The effect sizes were about the same at week 52, suggesting the volume loss was not progressive, said Reiman. Neither did it correlate with worsening total RBANS scores or with immediate or delayed recall.
Among 256 volunteers who had a follow-up MRI within two months of washout, their volume loss appeared at least partially restored. Cohen’s d values for drug/placebo comparisons of whole-brain and hippocampal volume had improved to -0.31 and -0.16, respectively. The difference in hippocampal volumes between drug and placebo groups was no longer significant.
Listeners wanted to know what caused the cognitive loss. Graf suspects umibecestat might have prevented BACE from cleaving substrates other than amyloid precursor protein, such as CHL1 or seizure protein 6 (Dec 2013 conference news; Oct 2016 conference news). She said that though Novartis has insufficient post-baseline CSF or PET data from Generation for further analysis, it is working on proteomics of CSF from previous three-month studies in healthy elderly volunteers to see if other BACE substrates might be affected and how that might relate to amyloid.
Others wondered how to interpret the loss of brain volume. Reiman believes it may reflect changes in the amount of brain fluid due to clearance of Aβ, an explanation proffered for other anti-Aβ therapies (Jul 2004 conference news; Dec 2017 news). In support of this, volunteers who were found to have elevated brain amyloid based on PET or CSF analysis had more brain shrinkage. “In general we are encouraged that we’ve seen only mild cognitive worsening, brain-volume changes that are not related to cognitive changes, and that all changes appear to reverse, or at least in case of MRI, begin to reverse,” said Reiman.
Umibecestat: Dead and Gone?
Graf told Alzforum that neither Novartis nor Amgen have plans for further development. Others believe BACE inhibitors may still prove useful. “We’ve got to think of the possibility that the drug deserves consideration for further use, as we begin to weigh the risks and benefits in future studies,” Reiman said.
Stefan Lichtenthaler, Technical University of Munich, agrees. “BACE inhibitors are promising, but need to be used at a lower dose (maximum 50 percent Aβ inhibition) and need to be given as a preventive drug and not for treatment of individuals already diagnosed with AD,” he wrote to Alzforum. He thinks 50 percent inhibition should be the max because mice missing one copy of the BACE1 gene appear normal.
Colin Masters, University of Melbourne, Australia, would dial the dosing down even further. Based on the rate of Aβ accumulation over a 20-year period, he believes 10 percent inhibition would be sufficient. He thinks the cognitive/psychiatric adverse events in Generation are due to umibecestat inhibiting the normal function of APP, which is needed for synaptic activity, learning, and memory. “The best strategy going forward, in my view, will be to use an antibody to lower the Aβ load to normal (might take a year or two) and then use a low-maintenance dose of a BACE inhibitor to keep it down,” he wrote to Alzforum.
If a BACE inhibitor is deployed in a future prevention trial, including a primary prevention trial in persons at genetic risk who do not yet have appreciable amyloid plaque deposition, then it will be important to consider ways to conduct the trial with adequate statistical power despite the modest worsening and very early preclinical stage, for example assessing persons with biomarker endpoints and/or with cognitive endpoints after temporary discontinuation of study drug,” Reiman wrote to Alzforum. “It will also be important to consider appropriate risk mitigation strategies.”—Tom Fagan
References
Therapeutics Citations
News Citations
- Cognitive Decline Trips Up API Trials of BACE Inhibitor
- In DIAN-TU, Gantenerumab Brings Down Tau. By a Lot. Open Extension Planned
- BACE—Substrates, Functions, Developmental Phenotypes
- BACE Inhibition and the Synapse—Insights from Seeon
- Philadelphia: Can a Shrinking Brain Be Good for You?
- Verubecestat Negative Trial Data: What Does it Mean for BACE Inhibition?
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.