It’s Not All About You, Neurons. Glia, Blood, Arteries Shine at Symposium
Quick Links
On April 4, 150 researchers gathered in Los Angeles at the University of Southern California’s Keck School of Medicine for the first Zilkha Symposium on Alzheimer’s Disease and Related Disorders. The conference aimed to put a growing neuroscience institute on the map as a regional research powerhouse while celebrating a generous donor. Unlike many events meant to feature an institution, the program was jam-packed with new data from researchers from around the United States, Europe, and USC. Together, they articulated the latest thinking around the theme of how cells other than neurons, genes other than APP or presenilin, and proteins other than Aβ might conspire to bring on Alzheimer’s. The discussions reflected how the field’s collective effort to understand neurodegeneration is subtly shifting away from two decades of neurocentric research and toward the brain’s glial and vascular cells—even cells and soluble factors from the blood. “Not everything that happens in the brain is about the neurons. Many systemic influences reach the brain, and the brain is the end organ that reacts,” said Berislav Zlokovic of USC. Zlokovic co-organized the meeting with Rudy Tanzi of Massachusetts General Hospital in Charlestown, Massachusetts, and David Holtzman of Washington University, St. Louis.
The meeting’s name is a nod to Selim Zilkha, the 87-year-old patron of USC’s Neurogenetic Institute in his name, which hosted the conference. An Iraqi-born businessman, Zilkha has thus far given $30 million to the institute and will receive an inaugural philanthropy prize in July at the Alzheimer’s Association International Conference in Copenhagen, Denmark. “I have started many businesses in my life, but my most important legacy is this institute. The quest is to find a way to prevent or arrest Alzheimer’s disease. It is not important which individual or institution succeeds, but it is critical that someone does,” Zilkha told the audience.
Titled “Breaking through Barriers: Neuronal, Glial and Vascular Contributions,” the conference focused on how vascular, perivascular, and radial, astro-, and microglial cells contribute to neurodegeneration. Alzheimer’s genetics discoveries have spurred molecular biology studies of the neurovascular unit. The meeting combined new insights in how genetic factors such as Trem2 or microRNA, as well as environmental risk factors such as mid-life hypertension, sleep, or aging, affect the cells in these systems.
No one doubts that the brain’s vasculature contributes to dementia, but a precise definition of its role, specifically in Alzheimer’s and other neurodegenerative diseases, is only beginning to come into focus. Both subtle dysfunction and outright physical damage of the blood-brain barrier were the topic of several talks. Specifically, researchers are scrutinizing what happens at the neurovascular unit. This is where blood and neurons come closest to each other deep inside the brain, after the larger arteries entering from the top have arborized into small arterioles and capillaries. These delicate micro vessels consist of a single layer of endothelial cells enwrapped by a single layer of contractile cells called pericytes. Pericytes themselves are covered by the end-feet of astrocytes, glia that also stand in direct contact with neurons. This in essence, constitutes the blood-brain barrier.
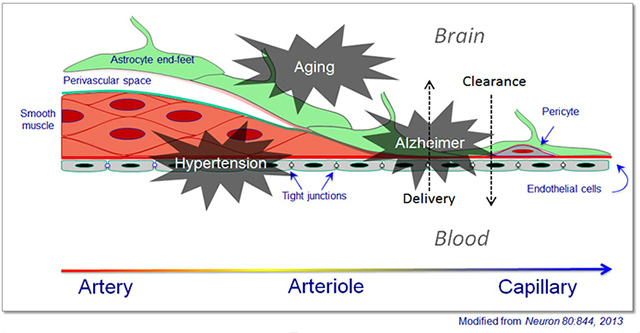
This diagram of a neurovascular unit shows how the cellular constituents of the walls of incoming arteries change as they dive deep into the brain and arborize to become arterioles, then capillaries.
Epidemiologic research has shown that high blood pressure in a person’s 40s, 50s, and 60s increases his or her risk for dementia and Alzheimer’s in late life. Why is that? Over the past five years, Costantino Iadecola of Weill Cornell Medical Center, New York, has been building a case that hypertension slowly disables the brain’s micro vessels, rendering them unfit to adjust blood flow to suit the brain’s needs. On the one hand, this cerebrovascular dysfunction exposes the brain to hypertension pressing in from the periphery, raising the risk of stroke. On the other hand, it impairs the brain’s ability to locally increase perfusion where the brain is most active, leading to cognitive decline.
“Hypertension is the curse of the brain,” Iadecola said, “Reduced cerebrovascular blood flow is directly related to mortality—it is as if the brain is running out of breath.” In a January 2014 Alzforum Webinar, Iadecola outlined the field’s current understanding of how immune factors and oxidative stress damage the neurovascular unit to reduce cerebral blood flow.
At the Zilkha conference, Iadecola focused squarely on the pathogenic mechanism of hypertension. Even a nudge up in systolic pressure—from 120 to 140 mmHg—has been reported to impair cognition (Knecht et al., 2008). Therefore, early consequences triggered during a person’s mid-life pre-hypertensive state need to be simulated in animals to understand the underlying mechanisms.
Slow-Pressor Model
Iadecola’s lab approximates this pre-hypertensive state by delivering the angiotensin II peptide hormone through mini-pumps implanted into mice. Small daily doses increase arterial pressure slowly, starting at one week. The scientists measure cerebral blood flow through a cranial window above the whisker barrel cortex. This allows them to increase local blood flow directly by applying vasodilators through the window, or indirectly by stimulating a given whisker. This so-called slow-pressor model of mild hypertension showed that cerebrovascular function in the brain becomes impaired in response to angiotensin even before arterial blood pressure has started to rise (Capone et al., 2011).
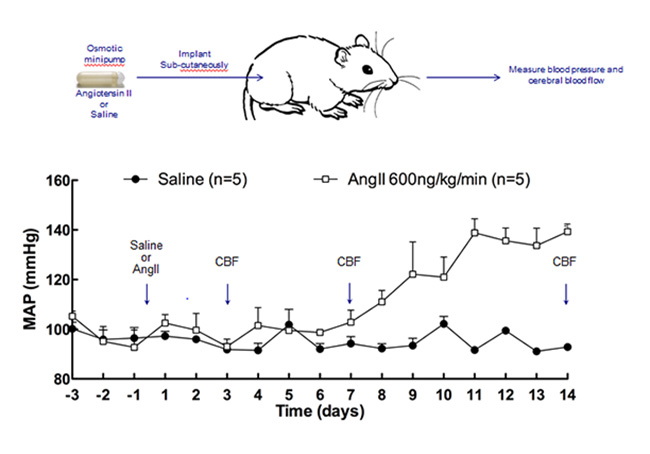
Repeated squirts of the hormone angiotensin II gradually nudge up blood pressure in an attempt to model the effects of mid-life hypertension on the brain. Arrows indicate time points when changes in cerebral blood flow are being measured. [Image courtesy of Costantino Iadecola.]
In new data, the electron microscope shows physical changes to the endothelial cells that line the brain’s blood vessels, including an increase in vesicular transport and permeability to low-molecular-weight dextran. The blood-brain barrier is compromised, and labeled angiotensin II penetrates through the vascular wall into pericytes and astrocytic processes. In behavioral assays commonly used in Alzheimer’s research, the slow-pressor mice underperform. “This suggest that even in the pre-hypertensive state, you might already have vascular damage in your brain,” Iadecola told the audience.
What mediates this early damage? At the conference, Iadecola showed a series of unpublished experiments pointing an accusing finger at the perivascular macrophage, a type of cell that patrols the spaces just outside the blood vessel. This macrophage binds angiotensin II with the angiotensin receptor on its surface and, in response, spews radical oxygen species. Three lines of experimentation to manipulate perivascular macrophages—pharmacological or genetic deletion, and bone marrow transplantation with macrophages lacking angiotensin II receptors—suggested that without these cells, slow-pressor angiotensin hypertension no longer caused cerebrovascular dysfunction.
“This work shows that hypertension damages endothelial cells and pericytes early on. It suggests hypertension could deteriorate your cerebral blood vessels very slowly, for some 20 to 40 years. That makes complete sense to me,” Zlokovic said.
Perhaps no talk at an Alzheimer’s conference is complete without an experiment connecting the pathway at hand to Aβ or tau, and Iadecola’s was no exception. He showed that inducing a pre-hypertensive state in Tg2576 mice brought on amyloid deposition in their micro vessels at an age when they would not ordinarily form plaques yet. “You give these mice angiotensin, their blood pressure goes up, and then they make more amyloid plaques,” Iadecola said. This finding echoes a recent human study that found elevated amyloid deposition in some people with hypertension (Rodrigue et al., 2013).
The larger implication is that treating midlife hypertension would protect against dementia. This was partially borne out in 2013, when several epidemiological studies reported that the incidence of dementia was edging down in several developed nations, and attributed this in part to better management of cardiovascular risk factors such as hypertension (see May 2013 news story, Jul 2013 news story).
Managing blood pressure as people age is not straightforward, however. That’s partly because high blood pressure is a risk factor in mid-life, whereas old people generally fare better on slightly elevated rather than low blood pressure. Numerous treatment studies have shown benefits for ACE inhibitors or beta-blockers, but results vary. “It is pretty clear that midlife hypertension should be controlled, but we have no strong convergent evidence to know which treatment is best,” said Helena Chang Chui, who heads the department of Neurology at USC. For more on the blood-brain barrier, see part two of this series.—Gabrielle Strobel
References
Webinar Citations
News Citations
- Dementia Incidence Said to Drop as Public Health Improves
- Dementia Prevalence Falls in England
- Fluid Markers and Imaging Back Idea of Breached Blood-Brain Barrier
Paper Citations
- Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, Berger K, Ringelstein EB. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008 Mar;51(3):663-8. Epub 2008 Feb 4 PubMed.
- Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H397-407. Epub 2010 Oct 22 PubMed.
- Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk Factors for β-Amyloid Deposition in Healthy Aging: Vascular and Genetic Effects. JAMA Neurol. 2013 May 1;70(5):600-6. PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.