CSF MTBR-tau-243 Tracks Tangles, Plummets in Response to Antibody
Quick Links
More than a decade before memory loss, fragments of phospho-tau start to rise in biofluids. These biomarkers, particularly p-tau217, have proven to be exquisite detectors of amyloid, but they plateau once symptoms surface and don’t track tightly with tau tangles. Here’s where MTBR-tau-243 comes in. According to a paper published July 13 in Nature Medicine, this particular fragment from tau’s microtubule-binding domain rises in step with tau-PET, and continues to climb even in the symptomatic stages of AD. Led by Randall Bateman of Washington University in St. Louis and Oskar Hansson of Lund University in Sweden, the study found that, more than any other tau biomarker tested so far, MTBR-tau-243 also correlates with cognitive decline, offering clinicians an important tool for both diagnosis and prognosis. What’s more, the putative tangle detector comes at a time when it’s sorely needed for tracking treatment responses in trials aimed at tau.
- In sporadic and familial AD, CSF MTBR-tau-243 tracks with tau-PET and cognition.
- p-Tau217 reflects amyloid, plateaus early in AD; MTBR-tau-243 continues to rise.
- In a handful of people thus far, MTBR-tau antibody halved CSF MTBR-tau-243.
Case in point, at the Alzheimer’s Association International Conference, held July 16-20 in Amsterdam, first data from early phase trials of Eisai’s E2814—an antibody that binds tau’s midsection—indicate that the drug safely engaged its target in people with familial AD. Importantly, it also cut CSF MTBR-tau-243 in half. Together, these findings hint at the possibility that finally, a tau antibody may be putting a dent tau tangles. Of course, the field still needs to find out if this target engagement stems cognitive decline.
In the AD brain, tau tangles comprise fibrils with a C-shaped protofilament core, which itself includes the third and fourth microtubule binding domains near the C-terminus of the protein (Jul 2017 news). Yet it is N-terminal fragments of phosphorylated tau, including p-tau181 and p-tau217, that are secreted by neurons in response to amyloid accumulation, making them sensitive fluid biomarkers for amyloid, not tau tangles (Mar 2018 news).
In search of chunks of tau in fluids that would signal the presence of tangles without the need for costly tau-PET scans, the WashU team previously identified MTBR-tau-243. Among a small number of participants in the Dominantly Inherited Alzheimer’s Network (DIAN), the researchers found that the mid-section fragment tracks with tau-PET and disease progression (Dec 2020 news).
The new study tests MTBR-tau-243 in two sporadic AD cohorts: Swedish BioFINDER-2, and the Knight AD Research Center. First author Kanta Horie, an Eisai-sponsored professor at WashU, and colleagues used mass spectrometry to measure MTBR-tau-243, as well as several phospho-tau markers, in the CSF of 448 BioFINDER-2 and 219 Knight ADRC participants. Both cohorts included people across the spectrum of AD, but BioFINDER-2 has a higher proportion of cognitively impaired people. Horie et al. asked which CSF markers tracked most closely with amyloid- and tau-PET. All phospho-tau markers were measured as a ratio of phosphorylated to unphosphorylated tau. When it came to tracking with amyloid-PET, p-tau217 emerged as the clear winner. For tau-PET, MTBR-tau-243 did the best. More than any of the p-tau species, MTBR-tau-243 tracked with tau-PET signal across all Braak regions.
Viewed from a different angle, the researchers found that amyloid-PET explained most of the variation in CSF p-tau217, while tau-PET best explained variability in MTBR-tau-243. In contrast, p-tau205 variability was equally accounted for by amyloid- and tau-PET.
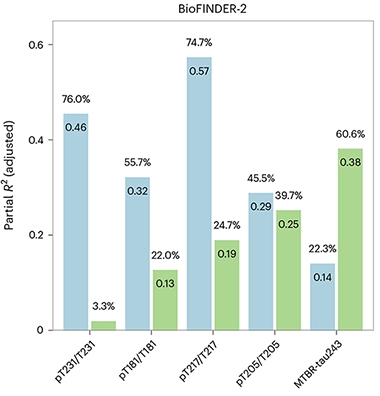
Pin the Tail on the Tangle. Percentage of variation in each CSF biomarker explained by amyloid-PET (blue) versus tau-PET (green). Most of the variation in p-tau231, 181, 217 was explained by amyloid; p-tau205 was equally explained by both. Tau-PET accounted for most of the variance in MTBR-tau-243 (right). [Courtesy of Horie et al., Nature Medicine, 2023.]
How would MTBR-tau-243 change as AD progressed? Among 220 participants in BioFINDER-2 who had serial CSF measurements, the scientists found that p-tau217, p-tau181, and p-tau231 increased dramatically when people became amyloid-positive, then plateaued when they became tau-positive. In contrast, MTBR-tau-243 hardly changed in response to amyloid positivity, but shot up once tau tangles had inundated the brain. The finding suggests that once a person becomes tau-PET positive, MTBR-tau-243 is the marker that best reflects their further disease progression. In line with this, MTBR-tau-243 tracked more closely than any other CSF biomarker with sinking scores on the mini-mental state exam (MMSE).
Finally, the researchers used an algorithm to pick out which combinations of fluid biomarkers best predicted amyloid accumulation, tau tangles, and cognitive decline. For amyloid, p-tau217 was the best single predictor; a combination of p-tau217, p-tau205, and Aβ42/40 did even better. For both tau-PET and MMSE, MTBR-tau-243 was the best single predictor. However, combining it with p-tau205 improved predictions for both measures. In fact, a combination of p-tau205 and MTBR-tau-243 rivaled tau-PET in predicting cognitive decline.
Hansson thinks the marker has both diagnostic and prognostic potential, particularly when used as part of a panel of fluid biomarkers for Aβ and tau. Because biomarkers such as p-tau217 rise many years before cognitive symptoms surface, and then plateau, a positive result leaves open the possibility that a person’s current cognitive impairment is caused by something other than AD, Hansson said. If MTBR-tau-243 was also elevated, this would give clinicians more confidence in calling AD the culprit. The marker will be most useful if detected in plasma, Hansson said, and researchers are working on that now.
Bateman agreed about the biomarker’s potential in the clinic. He also thinks it could help clinicians predict how well a given patient might respond to a therapy such as lecanemab, which worked better among people with low tau accumulation, as measured by PET, than among those with a high tau-PET signal. The same was true for donanemab, as reported at AAIC (see Part 1 of this series)
Gil Rabinovici of the University of California, San Francisco, noted that while convincing at the group level, the correlations between CSF MTBR-243 and tau PET are dogged by significant variability among individuals. “I think the utility of this biomarker for disease staging at the individual patient level is still to be determined,” he commented, adding that the impact of the new biomarker will depend on whether it can be detected in plasma. “With these caveats, MTBR-tau243 seems to be an interesting new biomarker, and it is especially exciting to see it utilized to measure target engagement in early phase clinical trials of MTBR-targeting tau antibodies,” Rabinovici wrote (comment below).
CSF MTBR-tau243 made its trial debut at AAIC, when Jin Zhou of Eisai presented findings from early-phase studies of E2814, a monoclonal IgG1 antibody that binds to the second and fourth microtubule-binding domains of tau. The researchers propose that the antibody intercepts seeding-competent, MTBR-containing fragments of tau released from cells, thwarting propagation of tau pathology.
Zhou showed findings from a multiple-ascending-dose study in 40 healthy participants, as well as results from a separate study conducted in people with dominantly inherited AD (DIAD). The latter included seven participants with mild to moderate dementia, who were slated to receive monthly infusions of E2814 over 18 months. Over that time, doses increased from 750 mg to 4,500 mg. The DIAD study is ongoing, and the remaining participants have reached the highest dose.
At AAIC, Zhou reported that the drug was safe and well-tolerated at all doses tested, in both healthy volunteers and those with AD. One person had two serious adverse events not attributed to treatment. Three of the seven participants in the DIAD trial dropped out due to cognitive deterioration, an expected problem in studies that include people with moderate dementia, Zhou told Alzforum. Among those with AD, target engagement appeared robust in the CSF, as the researchers detected dose-dependent binding of the antibody to both epitopes within tau’s MTBR.
Among the five participants with AD in which MTBR-tau-243 was measured, its concentration in the CSF plummeted in response to E2814 treatment. For four participants, it dropped between 40 to 70 percent within the first three months, in response to the lowest dose used. For one participant, the biomarker did not drop until dosing was doubled to 1,500mg; then it declined more slowly over the following months (see below).
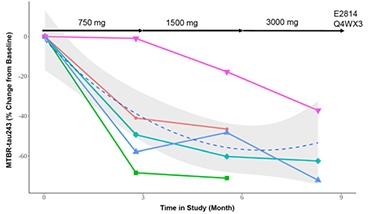
Taking Down Tangles? CSF-MTBR-tau243 dropped in response to treatment with E2814 in people with dominantly inherited AD. [Courtesy of Jin Zhou, Eisai.]
The biomarker findings suggest that E2814 lowered MTBR-tau-243, but did it knock down tangles? Only post-treatment tau-PET scans will tell. Zhou expects that scan data from the remaining participants within the next few months. “We are anxious and excited about that data,” she said.
Einar Sigurdsson of New York University believes the therapeutic potential of E2184 is well supported by its reduction of MTBR-tau-243. However, he noted that the majority of the antibody’s target is inside of neurons. “With this in mind, it would be interesting if the company examined whether the antibody is taken up into neurons, which would then greatly increase the pool of targetable tau,” he wrote. “If it is not, then it may be efficacious at a lower dose if its neuronal uptake could be enhanced, for example by altering its charge (Congdon et al., 2019).
Tiny single-domain antibodies are also being developed to target intracellular tau (May 2023 conference news). Sigurdsson added that an antibody’s ability to prevent tau seeding versus toxicity may not always go hand in hand. “It will be important to determine if this antibody and related ones against the MTBR region can reduce tau neurotoxicity in vivo, and reduce functional impairments,” he wrote.
E2814 is currently being evaluated in the DIAN-TU Tau NexGen trial, concurrently with lecanemab. The trial is running at 39 locations around the world, and expected to finish in 2027. Zhou said the new tangle-tracking biomarker comes at a perfect time, and the researchers are planning to integrate this measure into the ongoing DIAN trials. “There will be a cross-validation, for both the biomarker and the drug,” Zhou said.
Bateman said he expects the marker to be deployed broadly in clinical studies targeting both Aβ and tau. “If we start using it more across the trials, it will give us more information on this part of the pathology, so we can make better decisions based on what the drugs are doing.” For example, it could be possible that amyloid-targeted therapies that lower p-tau217 but not MTBR-tau-243 are less effective at slowing cognitive decline than drugs that lower both.
E2814 forms part of a field of MTBR-targeted therapies wending their way through preclinical and early clinical development. At AAIC, scientists from Prothena presented findings from the first in-human dosing of PRX-005. This human monoclonal antibody recognizes three epitopes within the first, second, and third microtubule-binding domains of tau. The single-ascending-dose study included 25 healthy participants, who received one of three doses of the antibody, or placebo. The drug appeared safe at all doses tested, and reached a sufficient concentration in CSF, suggesting it successfully crossed the blood-brain barrier. Currently, a multiple-ascending-dose study is ongoing in people with AD.
Prothena also reported preclinical findings on PRX-123, a dual peptide vaccine against Aβ and tau. The vaccine comprises the N-terminus of Aβ and the MTBR region of tau, complexed to an adjuvant that riles T-helper and B cells. At AAIC, Prothena reported that APP/PS1 mice immunized with PRX-123 produced a robust antibody response, and that antibodies in the mice's sera bound to both Aβ plaques and tau neurofibrillary tangles in brain sections from people with AD. The immunization also substantially lowered plaque burden in mice. —Jessica Shugart
References
Therapeutics Citations
News Citations
- Tau Filaments from the Alzheimer’s Brain Revealed at Atomic Resolution
- Isotope Labeling Links Tau Production to Aβ Burden
- MTBR-243 Tau: A Fluid Biomarker for Tangles Themselves?
- Donanemab Data Anchors Upbeat AAIC
- New Arrows Aimed at Tau: Single-Domain Antibody, Peptibody, Vaccine
Paper Citations
- Congdon EE, Chukwu JE, Shamir DB, Deng J, Ujla D, Sait HB, Neubert TA, Kong XP, Sigurdsson EM. Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy. EBioMedicine. 2019 Apr;42:157-173. Epub 2019 Mar 22 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Horie K, Salvadó G, Barthélemy NR, Janelidze S, Li Y, He Y, Saef B, Chen CD, Jiang H, Strandberg O, Pichet Binette A, Palmqvist S, Sato C, Sachdev P, Koyama A, Gordon BA, Benzinger TL, Holtzman DM, Morris JC, Mattsson-Carlgren N, Stomrud E, Ossenkoppele R, Schindler SE, Hansson O, Bateman RJ. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer's disease. Nat Med. 2023 Aug;29(8):1954-1963. Epub 2023 Jul 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCSF
The MBTR of tau represents a hot area of biomarker and drug development. Fluid biomarkers that target N-terminal epitopes (e.g., p-tau181, p-tau217, p-tau231) seem to be better markers for amyloidosis than tau pathology, while tau immunotherapy with N-terminal antibodies has led to disappointing results thus far in clinical trials. These findings have shifted the focus to the tau MTBR region, which may play a more important role in tau seeding and aggregation.
The paper by Horie et al. convincingly shows, across two independent cohorts, that CSF MTBR-tau243 is more strongly correlated with tau PET and clinical measures, and is a more dynamic longitudinal biomarker across the AD continuum than CSF N-terminal p-tau biomarkers. A fluid biomarker equivalent to tau PET could would have prognostic utility, aid with disease staging (as proposed in the recent update to the NIA-AA research framework), and (based on TRAILBLAZER-ALZ2 results) may predict response to anti-amyloid monoclonal antibody treatment.
While the correlations between CSF MTBR-243 and tau PET are convincing at the group level, there still seems to me to be significant variability between the CSF measure and tau PET at the single-subject level, so I think the utility of this biomarker for disease staging at the individual patient level is still to be determined. Ultimately, this may be better accomplished with a panel of fluid biomarkers than a single measure. Furthermore, the impact of this analyte would be greatly enhanced if it can be measured reliably in plasma in addition to CSF.
With these caveats, MTBR-tau243 seems to be an interesting new biomarker, and it is especially exciting to see it utilized to measure target engagement in early phase clinical trials of MTBR-targeting tau antibodies.
Washington University in St. Louis
The current paper, as well as work from 2021 by Dr. Kanta Horie exploring MTBR-tau243, demonstrates that it is the best fluid biomarker proxy of insoluble tau to date. He has also done fantastic work looking at other forms of MTBR in autosomal-dominant AD (Horie et al., 2023), as well as non-AD tauopathies (Horie et al., 2022).
This work opens up promising new markers for prognostics as well as an important readout in clinical trials. Alongside the work into different ptau phosphorylation sites led by Nicolas Barthelemy, we now have a richer characterization of changes occurring in tau from the very early signs of amyloidosis up the the formation of neurofibrillary tangles.
References:
Horie K, Barthélemy NR, Sato C, Bateman RJ. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer's disease. Brain. 2021 Mar 3;144(2):515-527. PubMed. Correction.
Horie K, Li Y, Barthélemy NR, Gordon B, Hassenstab J, Benzinger TL, Fagan AM, Morris JC, Karch CM, Xiong C, Allegri R, Mendez PC, Ikeuchi T, Kasuga K, Noble J, Farlow M, Chhatwal J, Day G, Schofield PR, Masters CL, Levin J, Jucker M, Lee JH, Roh JH, Sato C, Sachdev P, Koyama A, Reyderman L, Bateman RJ, McDade E, and the Dominantly Inherited Alzheimer Network. Change in Cerebrospinal Fluid Tau Microtubule Binding Region Detects Symptom Onset, Cognitive Decline, Tangles, and Atrophy in Dominantly Inherited Alzheimer's Disease. Ann Neurol. 2023 Jun;93(6):1158-1172. Epub 2023 Mar 16 PubMed.
Horie K, Barthélemy NR, Spina S, VandeVrede L, He Y, Paterson RW, Wright BA, Day GS, Davis AA, Karch CM, Seeley WW, Perrin RJ, Koppisetti RK, Shaikh F, Lago AL, Heuer HW, Ghoshal N, Gabelle A, Miller BL, Boxer AL, Bateman RJ, Sato C. CSF tau microtubule-binding region identifies pathological changes in primary tauopathies. Nat Med. 2022 Dec;28(12):2547-2554. Epub 2022 Nov 24 PubMed.
New York University School of Medicine
This Nature Medicine article nicely supports incorporating measurements of CSF levels of MTBR-tau as a potential biomarker for tau tangle pathology in AD. It is notable, though, that MTBR-tau in CSF was recently shown to be decreased in primary tauopathies by these same authors (Horie et al., 2022). This may complicate matters in mixed pathologies, and indicates that this epitope would have to be targeted intracellularly by therapeutic antibodies like E2814 in those non-AD tauopathies.
Regarding Jin Zhou's AAIC presentation, the safety profile and target engagement of E2814 look promising for ongoing clinical trials. Its therapeutic potential is also well supported by its reduction of MTBR-tau243 in CSF in the treated AD patients. This is not an artifact, because E2814 binds to aa 299-303 and to aa 362-366, whereas MTBR-tau243 consists of aa 243-254.
In Horie et al., 2021, tau fragments 299-317 and 354-369 showed a similar increase in AD patients as did fragment 243-254. This raises the question if these three fragments may be a part of a larger fragment in vivo that E2814 is then clearing. If this is not the case, then E2814-mediated clearance of MTBR-tau299 and MTBR-tau354 is somehow influencing clearance of MTBR-tau243. This might indicate an intracellular interaction where tau is less fragmented, whereas the clearance of the larger fragment could be intra- and/or extracellular.
With this in mind, it would be very interesting for the company to examine whether E2814 is taken up into neurons. This would greatly increase the pool of targetable tau, since most pathological tau is found inside neurons. Based on prior reports by some of these same investigators, most of extracellular tau consists of approximately amino acids 150-250, with MTBR-tau being only a minor fraction. Since extracellular tau is a tiny fraction of intracellular tau, therapeutic efficacy of tau antibodies is likely to be greatly enhanced if they can get into neurons.
We and other groups have shown that antibodies against tau and other targets can be taken up into neurons, and the degree of uptake depends at least in part on the charge of individual antibodies (Congdon et al., 2019; Congdon et al., 2022).
An AAIC poster by Sonia Talma et al. also spoke to this issue. These investigators had previously reported that the mouse version of E2814 reduced insoluble tau on the contralateral but not the ipsilateral side following brain injection of P301S tau seeds in P301S transgenic mice (Roberts et al., 2020). The effect was modest (around 30 percent reduction) although the antibody dose was high (40 mg/kg). However, treatment was acute (once a week for three weeks), which is more likely to reduce soluble tau though those levels were not reported.
On their poster presented at AAIC 2023, the authors report seeing greater efficacy of this antibody following a longer treatment period using AD tau seeds in htau transgenic mice, which better supports its clinical development. The dose required for efficacy remains rather high, though, with clearance of insoluble tau only seen at a 40 mg/kg dose but not at 3 or 10 mg/kg dose. The effective dose appears to be lower in the human study than in the mouse studies, although the measured parameters are different (CSF tau fragment vs. insoluble brain tau) so those studies cannot be directly compared.
Lastly, we have shown that an antibody’s ability to prevent tau seeding vs tau toxicity may not always go hand-in-hand (Congdon et al., 2019). It will be important to determine if this antibody and related ones against the MTBR region can reduce tau neurotoxicity in vivo and functional impairments. This has presumably already been examined, considering its advanced clinical development but it has yet to be published. We eagerly await that report and cognitive outcome of the ongoing AD trials.
References:
Horie K, Barthélemy NR, Spina S, VandeVrede L, He Y, Paterson RW, Wright BA, Day GS, Davis AA, Karch CM, Seeley WW, Perrin RJ, Koppisetti RK, Shaikh F, Lago AL, Heuer HW, Ghoshal N, Gabelle A, Miller BL, Boxer AL, Bateman RJ, Sato C. CSF tau microtubule-binding region identifies pathological changes in primary tauopathies. Nat Med. 2022 Dec;28(12):2547-2554. Epub 2022 Nov 24 PubMed.
Horie K, Barthélemy NR, Sato C, Bateman RJ. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer's disease. Brain. 2021 Mar 3;144(2):515-527. PubMed. Correction.
Congdon EE, Chukwu JE, Shamir DB, Deng J, Ujla D, Sait HB, Neubert TA, Kong XP, Sigurdsson EM. Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy. EBioMedicine. 2019 Apr;42:157-173. Epub 2019 Mar 22 PubMed.
Congdon EE, Jiang Y, Sigurdsson EM. Targeting tau only extracellularly is likely to be less efficacious than targeting it both intra- and extracellularly. Semin Cell Dev Biol. 2022 Jun;126:125-137. Epub 2021 Dec 9 PubMed.
Roberts M, Sevastou I, Imaizumi Y, Mistry K, Talma S, Dey M, Gartlon J, Ochiai H, Zhou Z, Akasofu S, Tokuhara N, Ogo M, Aoyama M, Aoyagi H, Strand K, Sajedi E, Agarwala KL, Spidel J, Albone E, Horie K, Staddon JM, de Silva R. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease. Acta Neuropathol Commun. 2020 Feb 4;8(1):13. PubMed.
Congdon EE, Chukwu JE, Shamir DB, Deng J, Ujla D, Sait HB, Neubert TA, Kong XP, Sigurdsson EM. Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy. EBioMedicine. 2019 Apr;42:157-173. Epub 2019 Mar 22 PubMed.
Make a Comment
To make a comment you must login or register.