Is ARIA an Inflammatory Reaction to Vascular Amyloid?
Quick Links
Can scientists who study Alzheimer’s disease find a solution to ARIA? These amyloid-related imaging abnormalities, which reflect brain edema, microbleeds, and occasionally large brain bleeds, have been associated with some deaths in clinical trials. At the Alzheimer’s Association International Conference, held July 16-20 in Amsterdam, scientists discussed the latest data on what causes ARIA.
- ARIA may be iatrogenic CAA-related inflammation.
- Anti-amyloid antibodies could trigger complement proteins to attack vascular amyloid.
- One TREM2 antibody causes ARIA as well.
- The hunt is on for biomarkers of CAA that could better predict ARIA risk.
They homed in on an inflammatory reaction to cerebral amyloid angiopathy. CAA experts noted that ARIA-E resembles CAA-related inflammation, a rare and serious condition caused by auto-antibodies to Aβ. In a plenary talk, Cynthia Lemere of Brigham and Women’s Hospital, Boston, sketched out a possible mechanism, suggesting that anti-amyloid antibodies may bind vascular amyloid, triggering the complement cascade to attack cerebral blood vessels. This would punch small holes, leading to fluid leakage and microhemorrhages.
Lemere and other speakers proposed therapeutic targets that might blunt the severity of ARIA, while highlighting the need for biomarkers of CAA to aid in prognosis and patient management (see Part 7 of this series). Pharma scientists noted that this is an active area of investigation at their companies, as well, with ongoing efforts to develop algorithms and blood tests to predict and better detect ARIA. “I’m hopeful we will be able to find a way around ARIA,” Lemere said in Amsterdam.
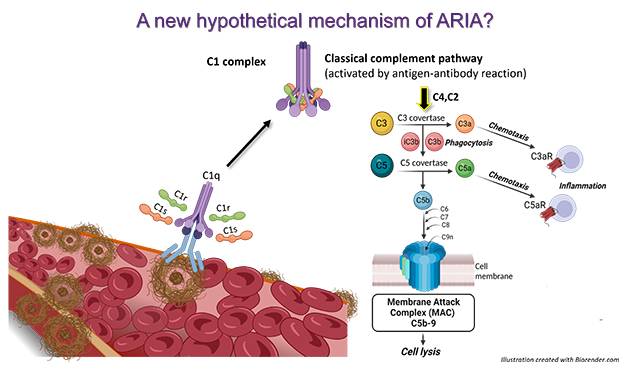
Complement As Hatchet Man. According to a new hypothesis, anti-amyloid antibodies (light blue) bind to vascular amyloid and trigger the complement cascade, prompting the membrane attack complex to form (dark blue), which damages blood vessels. [Courtesy of Cynthia Lemere and Maria Tzousi Papavergi.]
Vascular amyloid is common in AD. In Amsterdam, Roxana Carare of the University of Southampton, U.K., described how soluble Aβ in the parenchyma normally gets flushed out of the brain along blood vessels, draining into the basement membrane that packs the vessel wall. Excess Aβ can become trapped there, clogging the plumbing and forming CAA. White matter, which has fewer vessels and drains solutes more slowly than gray, is particularly vulnerable to this phenomenon. In postmortem analysis of brains that had CAA, fluid buildup can be seen in the perivascular space around white-matter vessels, damaging vascular architecture, Carare noted.
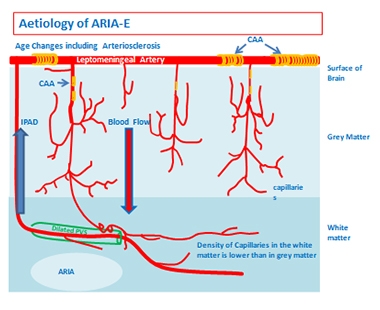
White Matter at Risk. Vascular amyloid (yellow) clogs leptomeningeal arteries and penetrating arterioles. Vascular damage (green) tends to be worse in white matter, which has slower blood flow. [Courtesy of Roxana Carare and Roy Weller.]
What happens during immunotherapy? Carare believes antibody therapy worsens the blockage, because antibody-antigen complexes become stuck in the already-obstructed basement membrane. In people immunized with Elan’s active vaccine AN-1792, the first attempt at immunotherapy 20 years ago, postmortem analysis revealed a worsening of CAA, along with damaged white matter (Boche et al., 2008).
Is Complement to Blame?
Lemere focused on how the innate immune system might contribute to ARIA. Praveen Bathini in her group investigated the effects of immunotherapy in 16-month-old APP/PS1 mice expressing human APOE4. These mice develop extensive vascular amyloid. Treating them for 15 weeks with 3D6, the mouse forerunner of the anti-amyloid antibody bapineuzumab, left cerebral vessels coated with the complement proteins C1q and C3. These vessels sprang leaks, leading to many microhemorrhages. In mice treated with a control antibody, vessels remained intact, as well as free of complement activation.
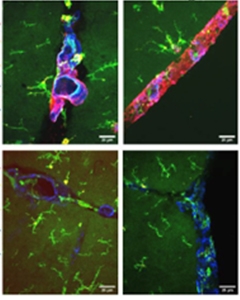
Antibodies Attract Complement. In amyloidosis mice treated with the 3D6 antibody (top), but not in those that receive an isotype control antibody (bottom), vascular amyloid (blue) in cerebellum (left) and meninges (right) becomes coated with complement protein C1q (red). The antibodies also attract activated microglia (green) and macrophages (gold). [Courtesy of Cynthia Lemere and Praveen Bathini.]
Lemere noted that after a patient has received an infusion of therapeutic antibodies, i.e., a big bolus entering their veins, these circulating anti-Aβ antibodies encounter and bind vascular amyloid first. She thinks these antibodies may get tagged by C1q, which recruits C1r and C1s, forming the C1 complex right there at the cerebral vessel wall. This complex would activate the classical complement cascade, producing C3a and C5a, and eventually resulting in formation of the membrane attack complex C5b-9. The complex pokes holes in cells, in this case along the blood-brain barrier, resulting in leakiness and microhemorrhages. She believes this may be the mechanism by which anti-amyloid antibodies give rise to ARIA-E and -H (see image at right).
In addition, complement proteins C3a and C5a likely activate microglia in the area, triggering vascular inflammation, Lemere said. Other talks in Amsterdam hinted at a microglial contribution, as well (see below).
If the complement cascade does trigger ARIA, then interrupting it, for example with antibodies directed against C1s, might halt this calamitous process. However, Lemere cautioned that therapies would have to be precisely directed, and brief, so as not to leave people immunocompromised. For example, while inhibiting C5 would disrupt the membrane attack complex, it would also hobble the body's essential antimicrobial defenses. C1q itself would also be a tricky target, because it promotes essential cellular function, such as differentiation, migration, and survival. Nonetheless, the complement cascade offers myriad targets, and Lemere said her group is exploring several of them.
These data caught the eye of pharma, with Rachelle Doody at Roche calling the findings an exciting lead. Roche is using data from its gantenerumab trials to model possible mechanisms of ARIA (Aldea et al., 2022).
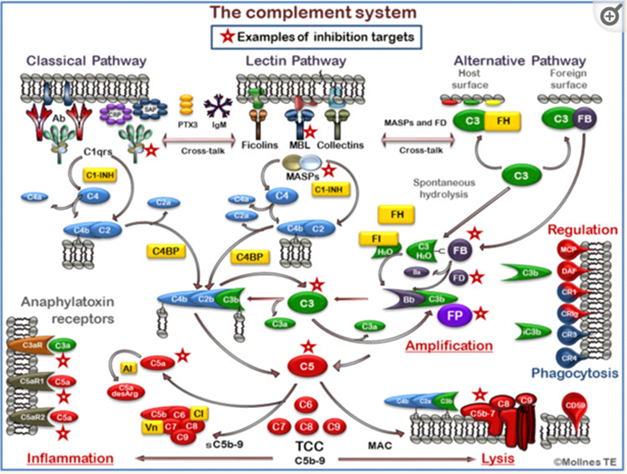
Myriad Targets. The complement cascade is complex, affording many points where therapeutic interventions could be aimed (red stars). [Courtesy of Garred et al., 2021.]
Is ARIA Treatment-Induced CAA-ri?
Others at AAIC elaborated on the links between ARIA and CAA-related inflammation (CAA-ri). Steven Greenberg of Massachusetts General Hospital, an expert on CAA, noted that they look suspiciously similar on MRI scans. Both conditions cause headaches and seizures. Both CAA-ri and ARIA tend to occur in APOE4 carriers, who have more vascular amyloid. Like ARIA, CAA-ri can trigger microbleeds, or occasionally larger hemorrhages. Most tellingly, CAA-ri events are associated with endogenous anti-amyloid antibodies, which peter out as the condition resolves (Kinnecom et al., 2007; Zedde et al., 2023). “ARIA-E is iatrogenic CAA-ri,” Greenberg concluded in Amsterdam.
Fabrizio Piazza of the University of Milano-Bicocca, Italy, linked Alzheimer’s to CAA-ri. He showed case studies of four patients with CAA-ri. Two, who were also positive for AD biomarkers, had more microglial activation, as seen by TSPO PET, in brain areas showing edema. The other two did not. With corticosteroid treatment, this microglial activation waned along with the edema and Aβ autoantibodies (Piazza et al., 2022). The data suggest there may be a heightened inflammatory response to CAA in the context of AD pathology, due to the disassembly of parenchymal plaques that floods clearance pathways with soluble Aβ, Piazza told Alzforum.
Notably, two of these four patients later developed microhemorrhages, seen at their five-month follow-up appointments. This jibes with findings from amyloid immunotherapy trials, where as many as 70 percent of these small bleeds occur after ARIA-E appears, Piazza noted (Salloway et al., 2022). By contrast, the occurrence of isolated ARIA-H, i.e., in the absence of ARIA-E, is the same in treated and control groups in all anti-amyloid trials to date. This suggests that it is the edema that amplifies the risk of microhemorrhages.
Piazza recommended treating ARIA-E with corticosteroids, such as methylprednisolone, as a way to prevent ARIA-H and larger bleeds. This would be a change from the current appropriate-use recommendations for immunotherapy, in which ARIA-E is only treated if serious symptoms develop.
Greenberg agreed, noting that in CAA-ri, several months of immunosuppressive treatment clears up the condition faster, with 94 percent of treated patients improved at the first follow-up appointment compared with half of untreated. In addition, only about a quarter of treated patients had another episode of CAA-ri within the next six years, compared with three-quarters of untreated (Regenhardt et al., 2020). For his part, Piazza found that stopping corticosteroid therapy abruptly, rather than tapering off, hikes the risk of CAA-ri recurrence, again pointing to the importance of careful management (Antolini et al., 2021).
CAA-ri also occurs in familial AD, said Natalie Ryan of the Dementia Research Centre at University College London. In Amsterdam, Ryan discussed the case of a woman with a presenilin 1 mutation, as well as two APOE4 alleles, who developed ARIA-E as seen on brain imaging, and who declined steeply. An autopsy confirmed the presence of CAA-ri (Ryan et al., 2015).
At AAIC, interest in learning how CAA-ri may relate to AD, and how it could inform ARIA management, was widespread. After all, the coming rollout of lecanemab, and perhaps also donanemab, will expose many more people to these antibodies than have been studied in trials.
Piazza runs the Inflammatory CAA and AD Biomarkers International Network. To date, iCAβ has enrolled more than 500 people with CAA-ri. The goal is to gather more data on this condition and use it to improve safety in immunotherapy trials.
Not Just Amyloid Immunotherapy?
One surprise at AAIC was that other AD immunotherapies can cause ARIA, too. Gary Romano, of the biotech Alector, presented data from the Phase 2 trial of Alector’s anti-TREM2 antibody in early AD. AL002 activates the TREM2 receptor, dialing up microglial activation and phagocytosis. To the researchers’ dismay, three participants, all APOE4 homozygotes, developed ARIA-E within the first three months of the trial. The condition resolved after halting dosing and treating the participants with corticosteroids.

E Before H. In the INVOKE-2 clinical trial with Alector’s Trem2 modulating antibody AL002, some participants developed MRI imaging abnormalities indistinguishable from ARIA-E and ARIA-H, just as in amyloid immunotherapy. [Proprietary images and information courtesy Alector.]
Alector has since changed its trial protocol to add MRI monitoring. The company now excludes E4 homozygotes, a more drastic measure that the anti-Aβ antibody programs have not taken. Alas, despite avoiding this highest-risk population, about a quarter of people on AL002 have developed ARIA-E or -H, with about one in eight cases being symptomatic. Romano showed two cases, one in an APOE4 heterozygote and one in a noncarrier, in which ARIA-E resolved within a few months, but was followed by microhemorrhages. This again mimics what happens with amyloid immunotherapy. The ARIA percentages are similar to those seen in those trials, as well.
It is unknown if the mechanism is the same, Romano said. However, the data are thought-provoking in light of Lemere and Piazza’s findings linking microglial activation to ARIA.
In the Clinic: Immunotherapy and ARIA
Initial clinical experiences with anti-amyloid antibodies reinforce the importance of careful management of ARIA. In Amsterdam, Matthew Howe, who works with Stephen Salloway at Butler Hospital in Providence, Rhode Island, presented data from the first 24 patients treated with aducanumab at Butler after its accelerated approval. Six developed ARIA-E, slightly fewer than the one-third who did in the Phase 3 trials. All six were APOE4 carriers, and three were homozygotes. Two of the six had symptoms of ARIA. For them, plus for two others who had ARIA deemed moderate to severe based on MRI, Howe stopped aducanumab permanently. For all six, their ARIA-E cleared up in about three months without corticosteroid treatment.
Despite the immediate stoppage, one initially asymptomatic patient, an E4 homozygote man, developed headaches a month later. Follow-up MRIs showed 18 new microhemorrhages scattered across his brain, well beyond the region of ARIA-E. His headaches and ARIA subsided with time, and he remained enthusiastic about trying new therapies. He has since enrolled in an anti-tau trial, Howe noted. The case illustrates that ARIA-E can have delayed effects. It also highlights the importance of halting dosing quickly for moderate ARIA, even when the person has no symptoms at the time of the MRI. Also at AAIC, Salloway reported that in the aducanumab trials, half of people with asymptomatic ARIA upon imaging went on to develop symptoms if dosing continued.
Lastly, in Amsterdam, researchers debated the link between anticoagulant use and higher risk of large brain bleeds in people on amyloid immunotherapy. In the open-label extension of the Phase 3 lecanemab trial, five people taking anticoagulants, or 3.6 percent of participants, had macro (not micro) hemorrhages. A macrohemorrhage is a bleed larger than 10 mm. Only 0.6 percent of people in the OLE who took lecanemab but no anticoagulants had macrohemorrhages, and only 0.2 percent of people on placebo in the original trial.
Greenberg called this risk substantial, noting that almost half of people who suffer a macrohemorrhage while on anticoagulants die within the next 30 days. Lecanemab’s appropriate-use recommendations warn against prescribing it to people on these medications (Jan 2023 news; Apr 2023 conference news).
Salloway noted that, so far, there is no evidence that donanemab increases the risk of macrohemorrhages for people on anticoagulants. Anticoagulants were allowed in donanemab’s Phase 3 trial, though the numbers were small, with 84 people taking both medications. For aducanumab, which is being given to some people who pay for it out of pocket, the AUR recommends against anticoagulant use. Aducanumab trials excluded the drugs. Every therapeutic antibody will get its own AUR, Salloway added.—Madolyn Bowman Rogers
References
News Citations
- Wanted: Fluid Biomarkers for CAA, ARIA
- Should People on Blood Thinners Forego Leqembi?
- Next Goals for Immunotherapy: Make It Safer, Less of a Hassle
Therapeutics Citations
Paper Citations
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008 Dec;131(Pt 12):3299-310. PubMed.
- Aldea R, Grimm HP, Gieschke R, Hofmann C, Lott D, Bullain S, Delmar P, Klein G, Lyons M, Piazza F, Carare RO, Mazer NA. In silico exploration of amyloid-related imaging abnormalities in the gantenerumab open-label extension trials using a semi-mechanistic model. Alzheimers Dement (N Y). 2022;8(1):e12306. Epub 2022 Jun 6 PubMed.
- Kinnecom C, Lev MH, Wendell L, Smith EE, Rosand J, Frosch MP, Greenberg SM. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007 Apr 24;68(17):1411-6. PubMed.
- Zedde M, Pascarella R, Piazza F. CAA-ri and ARIA: Two Faces of the Same Coin?. AJNR Am J Neuroradiol. 2023 Feb;44(2):E13-E14. Epub 2023 Jan 12 PubMed.
- Piazza F, Caminiti SP, Zedde M, Presotto L, DiFrancesco JC, Pascarella R, Giossi A, Sessa M, Poli L, Basso G, Perani D. Association of Microglial Activation With Spontaneous ARIA-E and CSF Levels of Anti-Aβ Autoantibodies. Neurology. 2022 Sep 20;99(12):e1265-e1277. Epub 2022 Aug 8 PubMed.
- Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, Suhy J, Forrestal F, Tian Y, Umans K, Wang G, Singhal P, Budd Haeberlein S, Smirnakis K. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022 Jan 1;79(1):13-21. PubMed.
- Regenhardt RW, Thon JM, Das AS, Thon OR, Charidimou A, Viswanathan A, Gurol ME, Chwalisz BK, Frosch MP, Cho TA, Greenberg SM. Association Between Immunosuppressive Treatment and Outcomes of Cerebral Amyloid Angiopathy-Related Inflammation. JAMA Neurol. 2020 Jun 22; PubMed.
- Antolini L, DiFrancesco JC, Zedde M, Basso G, Arighi A, Shima A, Cagnin A, Caulo M, Carare RO, Charidimou A, Cirillo M, Di Lazzaro V, Ferrarese C, Giossi A, Inzitari D, Marcon M, Marconi R, Ihara M, Nitrini R, Orlandi B, Padovani A, Pascarella R, Perini F, Perini G, Sessa M, Scarpini E, Tagliavini F, Valenti R, Vázquez-Costa JF, Villarejo-Galende A, Hagiwara Y, Ziliotto N, Piazza F. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology. 2021 Nov 2;97(18):e1809-e1822. Epub 2021 Sep 16 PubMed.
- Ryan NS, Lashley T, Revesz T, Dantu K, Fox NC, Morris HR. Spontaneous ARIA (amyloid-related imaging abnormalities) and cerebral amyloid angiopathy related inflammation in presenilin 1-associated familial Alzheimer's disease. J Alzheimers Dis. 2015;44(4):1069-74. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.