Meet the Artful Leftie: NIH Jump-Starts U.S.-Canadian FTLD Cohorts
Quick Links
On October 23, on the day the 9th International Conference on Frontotemporal Dementia got underway in Vancouver, Canada, the National Institutes of Health released news of three five-year grants totaling $5.9 million for longitudinal cohort studies and pathobiology research. While that may not seem like big money in some fields, in FTLD it is. “They are the largest grants the NIH has ever funded for frontotemporal dementia,” said Tony Phelps of the National Institute on Aging (NIA). The money is a welcome shot in the arm for U.S.-Canadian initiatives in presymptomatic and symptomatic FTLD, similar to such projects already underway in Europe (see Parts 2 and 3 of this series).
The NIA, with its sister organizations the National Institute of Neurologic Disorders and Stroke (NINDS) and the National Center for Advancing Translational Sciences (NCATS), put their heads and their wallets together to help separate, ongoing U.S.-Canadian FTD cohort studies coalesce in a larger way. The biggest slice, $3.4 million, goes to a U.S. version of the Genetic Frontotemporal Dementia Initiative (GENFI). The study is called the Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS—scientists pronounce it “Lefties”). Co-funded by NIA and NINDS, this multicenter study will be run by Bradley Boeve at the Mayo Clinic in Rochester, Minnesota, with Howard Rosen of the University of California, San Francisco, as co-principal investigator. The rest of the money is split about evenly. Adam Boxer at UCSF will lead a multicenter initiative to phenotype and genotype a broad-based cohort of FTLD. His team will characterize disease progression over one year, and build a consortium for therapeutic trials in such “readiness cohorts.” NINDS and NCATS co-fund this grant at $1.25 million. Thirdly, Leonard Petrucelli received $1.3 million from NINDS to explore, in his and other laboratories at the Mayo Clinic in Jacksonville, Florida, how C9ORF72 hexanucleotide repeat expansions cause FTD and ALS (see Part 7 of this series).
The LEFFTDS project engages seven centers in the United States, plus the University of British Columbia in Vancouver. LEFFTDS foresees longitudinal observation of 300 people in families who have inherited a pathogenic mutation in progranulin, C9ORF72, or tau. One hundred patients will be symptomatic mutation carriers, 100 will be asymptomatic mutation carriers, and 100 asymptomatic mutation non-carriers. “With LEFFTDS we are now studying asymptomatic mutation carriers, and do so specific to their mutation. We have never done that before,” said David Knopman, also at Mayo in Rochester.
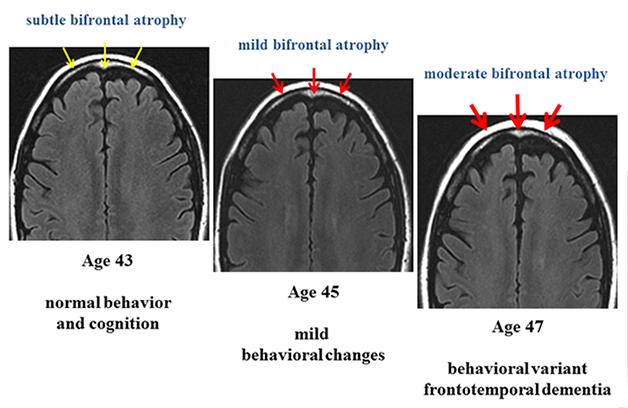
Longitudinal MRI scans of a person carrying a pathogenic mutation in the gene encoding the tau protein, who becomes symptomatic during this time. [Image courtesy of Bradley Boeve, Mayo Clinic.]
Each of the participating sites already follow autosomal-dominant cases of FTD; indeed, all envisioned LEFFTDS participants are known to the centers, though if more come forward they will be allowed in, Boeve told Alzforum. Between the centers, the researchers currently work with 835 people from 306 families. About 100 are asymptomatic mutation carriers; an additional 300 asymptomatic relatives remain to be genotyped. LEFFTDS will enroll people starting in their 30s and invite them to their respective center once a year; it is currently funded for three such visits.
This study will use clinical, neuropsychological, biofluid, and neuroimaging measures, though not tau PET at present. As is becoming apparent in GENFI, LEFFTDS researchers also expect that they may see patterns of sequential change in those markers that are specific to each causative gene. Unlike in autosomal-dominant Alzheimer’s, the three main FTD genes are not known to impinge on the same molecular pathway. Much like in TrackHD, the Dominantly Inherited Alzheimer Network (DIAN), and GENFI, the overarching twin goals of LEFFTDS are to understand how the underlying proteinopathy causes disease and to determine how future disease-modifying trials can measure whether a drug is effective and engaging its target.
The second grant is called Advancing Research and Treatment for Frontotemporal Lobar Degeneration, or ARTFL. (Unsure how to pronounce this new acronym? Try “Artful”.) Previously this was called the FTLD Clinical Research Consortium. The overall goal here is to promote more therapeutic trials. From its headquarters at UCSF, ARTFL is working with six advocacy groups: the Association for Frontotemporal Degeneration (AFTD), the Bluefield Project, the Tau Consortium, CurePSP, CBD Solutions, and the Alzheimer’s Drug Discovery Foundation (ADDF). ARTFL engages the LEFFTDS sites plus six more, for a total of 14 sites across the United States, Toronto, and Vancouver.
ARTFL is designed to funnel an initially broad range of about 1,500 participants with any disease across the FTLD spectrum into a registry that is open to the public. From there, ARTFL will select about 900 participants for thorough characterization to build what are increasingly referred to as “readiness” cohorts for therapy trials. This includes a baseline workup of clinical, neuropsychology, imaging, and fluid markers for selected populations in whom the scientists believe they can determine which underlying proteinopathy drives their clinical disease. Moreover, ARTFL envisions a project to follow disease progression closely for one year, the time future FTD efficacy trials are expected to last. This serves to define progression scales and outcome markers. Finally, ARTFL includes genotyping for gene discovery; those participants in whom an autosomal-dominant mutation crops up would be offered a spot in LEFFTDS and/or in therapy trials for gene-specific subtypes of FTLD, said Boxer.
With GENFI, LEFFTDS, ARTFL, and the German and other networks, there are now a number of FTD consortia across the Atlantic Ocean. GENFI2, LEFFTDS, and ARTFL each expect to start up in spring 2015 after spending the winter preparing sites and obtaining permission of institutional review boards, etc.
The projects’ leaders each profess interest in making the data from their respective initiatives compatible to pooled analysis. Beyond the obvious lure of gaining greater statistical power for research on a rare disease, coordination has other upsides. For example, by sharing demographic, clinical, and genetic data internationally, centers can build better pedigrees. “If we are following siblings and cousins in the U.S., and a branch in Europe turns out to descend from the same founder mutation, then we can base age of onset on more data,” Boeve told Alzforum. Knowing when a mutation carrier will become symptomatic is important to decide when to start treatment, and to determine if putative disease-modifying agents delay onset. To link participant data across continents in a secure way, Boxer suggested the different studies set up a global unique identifier system (GUID), a code that would work much like a social security number for FTD.
A global, well-characterized patient population would attract more companies to developing therapeutics for this indication. It might also enable FTD researchers to act as a unified group in negotiating with industry, the study leaders agreed. “We want to assure that we have good publications coming out of the trials, and that data and biospecimens will be shared so that we can advance the science,” Boxer said. Toward this goal, Jonathan Rohrer of University College London, who co-leads GENFI, suggested that the study leaders meet periodically to exchange what they learned. He offered an acronym—FPI, for FTD Prevention Initiative—and extended an invitation to meet in Europe in summer 2015.
In 2011, DIAN created a pharma consortium with more than a dozen member companies that jointly fund trial preparation research and meetings. The consortium companies nominate their drugs for clinical trials in the DIAN population, but an independent committee of clinician-researchers then makes the choice (Dec 2011 news story). In addition, the leaders of DIAN, the Alzheimer’s Prevention Initiative (Feb 2010 news story), and the A4 study founded a group called CAP (aka Collaboration to Prevent Alzheimer’s), which meets several times a year to coordinate assessments and procedures as closely as possible (Aug 2012 news story).
As another area of global collaboration, Boxer suggested a jointly funded global patient-research registry. This might work well across international borders if it was built as a federated platform where certain core information could be merged but other components are maintained locally in separate, national registries, Boxer said at ICFTD. Prior examples include the Global Dystonia Registry, the Brain Health Registry started at UCSF, and the Alzheimer’s Prevention Registry.
Goodwill notwithstanding, there are limits to cross-Atlantic cooperation. For example, in its effort to nudge the North American studies to collaborate and contribute to existing data platforms in Alzheimer’s disease, the NIH likes to see its funded projects deposit de-identified data in databases such as the database of Genotypes and Phenotypes (dbGaP). ARFTFL and LEFFTDs will also upload their imaging data to the place where ADNI data lives, the Laboratory of Neuro Imaging (LONI) at the University of Southern California. Moreover, both initiatives will use the NACC-FTLD test module. This was created as an outgrowth of the Unified Data Set collected as part of the U.S. Alzheimer Disease Research Centers system, and the tests are available for free without licensing fees (May 2012 news story). For its part, GENFI uses an overlapping but not identical set of tests and translates them into many languages. The NIH also requires that ARTFL and LEFFTDS contribute blood and other biospecimens to the National Cell Repository for Alzheimer’s Disease (NCRAD) and the NINDS Coriell Repository, which collect information from NIH-funded studies for use in neurogenetics and other research.
ARTFL and LEFFTDS nabbed their new funding in part because they had been laying the groundwork in smaller projects. On those, the ICFTD featured various data updates. For example, the Neuroimaging in Frontotemporal Dementia project is an imaging study in symptomatic FTD. The NIFD is in the midst of collecting second scans on 74 people with bvFTD, 34 with svPPA, 33 with nonfluent PPA, and 73 controls. In his talk, Rosen of UCSF said that he expects the regions that atrophy in a given patient will predict which symptoms that person develops later, and that the study can define rates of atrophy in specific brain areas against which to measure future drug effects.
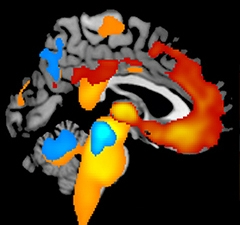
This image shows where MRI scans taken one year apart in two types of FTLD measured the greatest loss of brain tissue. Red-yellow areas indicate the relative severity of atrophy in people with behavioral variant frontotemporal dementia (bvFTD), whereas yellow-orange-blue areas show annual tissue loss in progressive supranuclear palsy (PSP). Color images superimposed on MRI of an healthy adult brain. [Image courtesy of Shubir Dutt, Howard Rosen, Adam Boxer, UCSF.]
Already, short stretches of one-year longitudinal data suggest that atrophy in defined areas starts presymptomatically, accelerates when symptoms set in, and bottoms out in the overt symptomatic phase, Rosen said. In essence, the FTLD field is beginning to validate theoretical biomarker staging diagrams with empirical data from longitudinal observation, as Alzheimer’s research has been doing for some years. With the new grants, several of these studies can now band together for a better-funded continuation of their ongoing work.
Rosen compares his site’s data with those of Brad Dickerson at Massachusetts General Hospital, to see if the two sites come up with similar results. “We are very close,” Rosen said in Vancouver. The combined data serve to plan future treatment trials. For example, analyzing reams of existing scans across the two sites has led to a realization that FDG-PET—an established technique that infers brain activity in a given region via that region’s glucose use—may not be worth the expense in large FTD trials, Rosen claimed. Brain maps made by various MRI techniques, such as arterial spin labeling, are proving as good. “There may not be added value of FDG PET over MRI,” Rosen said.—Gabrielle Strobel
References
News Citations
- First Data from GENFI1: Brain’s Insula Region Shrinks A Decade Before FTD
- German Network of 700 FTLD Patients Presents Baseline Data
- Cloak and Dagger Clusters? How C9ORF72 Repeats Kill Is Still a Mystery
- DIAN Forms Pharma Consortium, Submits Treatment Trial Grant
- Phoenix: Vision of Shared Prevention Trials Lures Pharma to Table
- Collaborative Umbrella CAPs Three Prevention Trial Initiatives
- NACC News: Building a Frontotemporal Database, and More
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.