Ready or Not: Stem Cell Therapies Poised to Enter Trials for Alzheimer’s
Quick Links
Stem cells have been hailed, and hailed some more, as a breakthrough technology. All the same, they have been slow to make real inroads in the understanding and treatment of Alzheimer’s disease. That is about to change, according to scientists who spoke at “Accelerating the Cure for Alzheimer’s Disease through Regenerative Medicine.” Held November 6 at Duke University, Durham, North Carolina, the symposium was co-chaired by Murali Doraiswamy and Joanne Kurtzberg. Kurtzberg is a pediatrician and cell therapy expert at Duke.
The first clinical trials of stem cells for AD are expected to begin in 2015, speakers said. Some cautioned that many questions remain about how stem cells affect the Alzheimer’s brain. They debated whether the move into the clinic is premature, noting the need for more research into where in the brain stem cells go and how long they last. On this, attendees were intrigued by some success tracking injected cells with MRI. In addition to therapeutic applications, induced stem cells made from patients with AD and related disorders are helping shed light on disease mechanisms and enabling screens for potentially therapeutic compounds. Research on stem cells remains limited, however, in part because it is largely supported by state initiatives, such as the California Institute for Regenerative Medicine, and private foundations, such as the New York Stem Cell Foundation.
Can Stem Cells Treat Alzheimer’s?
Therapies based on stem cells have had some success in other diseases. Transplants of cord blood, for example, are approved by the Food and Drug Administration to treat leukemia and inborn errors of metabolism, Kurtzberg said in a talk in which she described treatments pioneered by the Duke Stem Cell and Regenerative Medicine Program. Clinicians irradiate the patient’s bone marrow before administering cord blood, then stem cells in the donated blood engraft and replace unhealthy cells. In the last 25 years, more than 35,000 such life-saving transplants have taken place, Kurtzberg noted.
In Alzheimer’s disease, however, neurons die off in massive numbers throughout the brain, making cell replacement impractical. This has caused many researchers to overlook stem cell therapy as an option for AD, Mahendra Rao said in his keynote address. Rao leads regenerative medicine at the New York Stem Cell Foundation Research Institute, New York City. Nonetheless, stem cells have demonstrated the ability to improve cognition in animal models. Rather than replacing neurons, they may benefit the brain in other ways, such as by modulating inflammation, stimulating remyelination, and supplying trophic support. “This may enhance the life of dying neurons,” Rao said. Would injected cells succumb to the surrounding disease, as has been found to happen with fetal neuron grafts in Parkinson’s patients (see Apr 2008 news story; Jun 2014 news story)? Quite the opposite, Rao believes. Injected stem cells, which often mature into glia, may help modify the brain environment, thus lowering its toxicity.
One example of how stem cells can promote neuron health came some time ago from Frank LaFerla of the University of California, Irvine. LaFerla injected mouse neural stem cells into the brains of animals that modeled hippocampal sclerosis and Alzheimer’s disease. Treated mice improved cognitively but, in synch with what Rao said, injected cells neither became neurons nor lowered Aβ or tau pathology. Instead, they promoted formation of synapses in the CA1 region of the hippocampus, an effect LaFerla traced to stem cells releasing brain-derived neurotrophic factor (BDNF). Knocking down this growth factor in the cells before injecting them abrogated the treatment benefit (see Jul 2009 news story). Could stem cells slow disease progression? When LaFerla modified neural stem cells to express the Aβ-degrading enzyme neprilysin, treatment with these cells lowered Aβ deposits throughout mouse brain (see Aug 2010 conference story). It is not clear if this step improves cognition further, LaFerla said in answer to an audience question.
Stem cells may also replace sick glia. Kurtzberg described the use of cord blood stem cells to treat Krabbe’s disease in children. This genetic disorder is caused by the lack of the enzyme galactosylceramidase, which helps maintain the myelin wrapped around axons. Babies born with this condition develop muscle weakness and seizures, and most die before they are 2 years old. However, those who receive cord blood transplants around one month of age, before symptoms appear, survive and generally thrive, with only mild development delays, Kurtzberg said (see Escolar et al., 2005).
Most of the stem cells that engraft in the brains of these children become glia. Studying engrafted cells in postmortem samples, Kurtzberg’s group found that they shared properties of microglia and oligodendrocytes. In mouse models, these cells secrete soluble anti-inflammatory factors, stimulate neurogenesis, and promote remyelination. The researchers are now generating these cells in vitro and are about to begin a Phase 1 trial to test whether delivering them into cerebrospinal fluid, in addition to the standard cord blood transplant, is safe in children with Krabbe’s disease. The differentiated cells should engraft more quickly than do stem cells, which take months to provide the full benefit, and thus may improve outcomes, Kurtzberg noted.
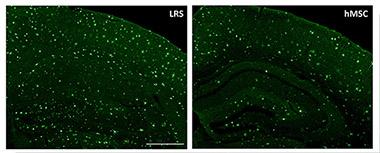
Cleanup Crew?
Amyloid deposits (green) in cortex and hippocampus of mice treated with human mesenchymal stem cells (right) are reduced by one-third compared to untreated mice (left). [Image courtesy of Alexei Lukashev and Tristan Bolmont, Stemedica International.]
In other cases, it is not yet clear what the stem cells do. Researchers led by Alexei Lukashev of the biotech company Stemedica International, Epalinges, Switzerland, presented a poster on the use of human adult mesenchymal stem cells to combat Alzheimer’s disease. First author Tristan Bolmont injected the cells into the bloodstream of 15-month-old APP/PS1 mice once per week for 10 weeks. The amyloid load in the hippocampus of treated mice dropped by one-third compared with untreated controls. Meanwhile, more microglia clustered around plaques, while the number of pro-inflammatory microglia shrank, suggesting that the stem cells somehow influence this balance. In ongoing work, the authors are characterizing this, as well as testing behavior in treated mice. The cells appeared safe, with no increase in vascular amyloid or microhemorrhage.
Human mesenchymal stem cells are currently in a Phase 1/2 clinical trial for stroke. Lukashev said he is applying for FDA approval for a Phase 2 Alzheimer’s study to start next year. Similar approaches by other groups are in trials for multiple sclerosis and amyotrophic lateral sclerosis (ALS) (see Oct 2010 news story).
Other stem cell-based Alzheimer’s treatments are also on the threshold of the clinic. Ellen Feigal, who leads research and development at the California Institute for Regenerative Medicine (CIRM), noted that her organization has given out 17 awards for Alzheimer’s projects; three of those are now applying for FDA approval for trials. Two academic studies identified small neuroprotective molecules through screens of stem cells; the third, led by Alexandra Capela at the biotech company StemCells Inc., Newark, California, proposes to transplant neural stem cells into AD patients (see CIRM award).
Conference attendees disagree whether the technology is ready for human study. “We are rushing too fast to the clinic,” LaFerla cautioned, noting that many questions of basic science remained to be answered. Also, researchers do not yet know how many cells to deliver, where, and how often in order to optimize the response, he said. Bolmont conceded that questions remain, but said he felt many of them can be answered only by trials. “We could do two more years of mouse studies, and we would still have the same question about whether these treatments will work in humans,” he told Alzforum. Feigal urged that trials and research run in parallel. “We don’t need to paralyze trials while answering basic questions. Clinical trials can inform research. It is a two-way street,” she said.
Thomas Finn from the FDA spelled out potential safety concerns for which his agency will watch. They include whether intravenous injections of stem cells might block capillaries, causing embolisms and damaging brain tissue. Another question is whether stem cells might give rise to tumors, or make the wrong kind of cells or connections in the brain, leading to side effects like chronic pain.

Where Did the Injection Go?
Stem cells labeled with the MRI agent SPIO spread through one brain hemisphere after injection into a carotid artery. [Image courtesy of Piotr Walczak, Miroslaw Janowski, Jeff W.M. Bulte, et al.]
Tracking Cells in Vivo
To allay these concerns, researchers want to be able to follow injected stem cells to see where they go and what kind of cells they become. In his talk, Jeff Bulte, a radiologist at Johns Hopkins University, Baltimore, discussed ways to label stem cells with contrast agents so that MRI can tracked them (see Ahrens and Bulte, 2013). One such agent, superparamagnetic iron oxide (SPIO), is sensitive and appears safe, having been used in several clinical trials. In a human case study, SPIO maintained a signal for more than four months (see Janowski et al., 2014). However, this compound has been pulled from the market for economic reasons, Bulte told Alzforum.
Probes containing the stable isotope fluorine-19 (19F) emit a bright signal but are less sensitive than SPIO (see Ruiz-Cabello et al., 2008). Celsense Inc. of Pittsburgh markets these probes, which are in use in at least one clinical trial. Bulte’s own company, SenCEST LLC, Fulton, Maryland, develops chemical exchange saturation transfer (CEST) agents. Instead of using metal, the exchange of a proton between the agent and surrounding water creates the signal. All three approaches demonstrate potential for human use at this time, Bulte said.
With all these labels, however, the signal fades over time. Bulte is experimenting with reporter genes that could give long-term information on injected cells, with the added advantage of turning off when cells die. As an example, he said that stem cells can be engineered to express a thymidine kinase from herpes simplex virus, which is then detected by a thymidine analogue probe using CEST (see Bar-Shir et al., 2013; Bar-Shir et al., 2013). Bulte also found that stem cells can be targeted to areas of inflammation by making them express the docking protein VLA-4 on their cell surface. This molecule binds to VCAM-1, which is expressed by inflamed endothelial cells. This way, stem cells penetrate into the brain three- to fourfold better, Bulte claimed (see Gorelik et al., 2012).
Such tracking technologies provide a glimpse into the life of injected cells. Using MRI labels, Bulte found that stem cells injected into carotid arteries fanned out across the brain more broadly than cells injected into the brain’s ventricles or parenchyma (see image above). In an animal study, injection of large cells by this route did not disrupt the integrity of blood vessels in the brain, Bulte said.
Modeling Disease: What if Craig Had an Alzheimer Mutation?
Short of treating disease, researchers hope that stem cells will at least model human disease more faithfully than do animals or cell lines. Lawrence Goldstein of the University of California, San Diego, previously reported that induced neurons generated from people with APP mutations produced loads of Aβ40 and phosphorylated tau (see Jan 2012 news story). At Duke, Goldstein noted that genetic variability between individual people poses a problem for modeling disease with induced pluripotent stem (iPS) cells. To address this, he generated iPS cells from a person whose genome was fully sequenced: genetics pioneer Craig Venter (see Nov 2008 news story). Then he introduced mutations in either APP or presenilin and compared the results. To his surprise, presenilin mutations did not boost phosphorylated tau, while APP mutations did. Why this difference? Goldstein does not know yet. Currently, he is culturing induced Venter neurons with astrocytes to see if cellular interactions might influence tau processing.
Neurons in iPS cells can model other neurodegenerative diseases as well. Chris Henderson of Columbia University, New York City, generated induced corticospinal motor neurons, which selectively degenerate in ALS. Henderson compared their gene expression profile to that of oculomotor neurons, which remain intact throughout the disease, to learn what makes spinal motoneurons so vulnerable. He turned up 15 candidate susceptibility genes and four potential resistance genes. When he knocked out one of the susceptibility genes, matrix metalloproteinase-9 (MMP-9), from ALS mouse models, they lived 80 days longer than littermate controls, extending their lifespan by about one-quarter. MMP-9 is expressed almost exclusively by motor neurons and might make an attractive therapeutic target, Henderson suggested (see Kaplan et al., 2014).
Researchers also use induced neurons to screen drugs. Goldstein screened a library of 3,000 compounds on induced neurons from AD patients. He found four classes of drug that lowered Aβ, and said he is working on advancing some of them to the clinic. Henderson screened 50,000 compounds on induced motor neurons to find some that stimulated axon growth in an inhibitory environment. Surprisingly, statins boosted axon extension the most, leading to a 30-fold increase in growth. Henderson believes that the drugs are acting via a mechanism other than cholesterol. Statins themselves would not make good ALS therapeutics because they barely enter the brain and have systemic side effects, he noted. He is looking for other ways to stimulate the same pathway.
Attendees debated whether stem cells will lead to personalized medicine. In theory, clinicians could use iPS cells to generate replacement cells that contain a patient’s own DNA. By studying iPS cells from multiple donors, researchers could find specific genetic factors that predict whether a given patient will respond to a particular therapy. Clinicians want these options, speakers said, but pharmaceutical companies protest that such approaches would not be commercially viable. In practice, stem cell therapies will not attempt genetic matching in the foreseeable future, clinicians agreed. The question of whether autologous injected stem cells will last for a lifetime without inflaming the immune system will likely be answered sooner.—Madolyn Bowman Rogers
References
News Citations
- Dopaminergic Transplants—Stable But Prone to Parkinson’s?
- Fetal Dopamine Grafts for Parkinson’s Remain Healthy After a Decade
- Support Cast: Neural Stem Cell BDNF Prompts Memory in AD Mice
- Honolulu: Neuroregeneration Taps Glia, Neurotrophins
- Stem Cells Pass Initial Safety Muster in ALS, Multiple Sclerosis
- Induced Neurons From AD Patients Hint at Disease Mechanisms
- With Two More Completions, Personal Genomics Picks up Speed
Research Models Citations
Paper Citations
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005 May 19;352(20):2069-81. PubMed.
- Ahrens ET, Bulte JW. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 2013 Oct;13(10):755-63. Epub 2013 Sep 10 PubMed.
- Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, Lukomska B, Roszkowski M. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS One. 2014;9(2):e97631. Epub 2014 Jun 11 PubMed.
- Ruiz-Cabello J, Walczak P, Kedziorek DA, Chacko VP, Schmieder AH, Wickline SA, Lanza GM, Bulte JW. In vivo "hot spot" MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med. 2008 Dec;60(6):1506-11. PubMed.
- Bar-Shir A, Liu G, Liang Y, Yadav NN, McMahon MT, Walczak P, Nimmagadda S, Pomper MG, Tallman KA, Greenberg MM, van Zijl PC, Bulte JW, Gilad AA. Transforming thymidine into a magnetic resonance imaging probe for monitoring gene expression. J Am Chem Soc. 2013 Jan 30;135(4):1617-24. Epub 2013 Jan 16 PubMed.
- Bar-Shir A, Liu G, Greenberg MM, Bulte JW, Gilad AA. Synthesis of a probe for monitoring HSV1-tk reporter gene expression using chemical exchange saturation transfer MRI. Nat Protoc. 2013 Dec;8(12):2380-91. Epub 2013 Oct 31 PubMed.
- Gorelik M, Orukari I, Wang J, Galpoththawela S, Kim H, Levy M, Gilad AA, Bar-Shir A, Kerr DA, Levchenko A, Bulte JW, Walczak P. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology. 2012 Oct;265(1):175-85. Epub 2012 Aug 24 PubMed.
- Kaplan A, Spiller KJ, Towne C, Kanning KC, Choe GT, Geber A, Akay T, Aebischer P, Henderson CE. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron. 2014 Jan 22;81(2):333-48. PubMed.
External Citations
Further Reading
News
- Stem Cells Grown From 86-Year-Old Man Morph into Neurons in Rat Spinal Cord
- Stem Cell Screen Points to ALS Disease Target
- In-Vitro Innervation: Stem Cell-Derived Motor Neurons Meet Muscles
- Editing Stem Cell DNA to Make the Perfect Cells for Study, Therapy
- Turning Human Fibroblasts Into Neurons; Making Safer Stem Cells
- In Alzheimer Disease Research, iPS Cells Catch On Slowly
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.