Do Astrocyte Receptors Go Over the Top in Alzheimer’s?
Quick Links
Astrocytes perform an elaborate array of support functions for neurons, but if they get out of sorts they can lead to trouble. At the Society for Neuroscience 2014 Annual Meeting, held November 15 to 19 in Washington, D.C., researchers reported on two receptors that might pack a punch in astrocytic cell membranes in Alzheimer’s disease. Astrocytes near plaques overexpress the P2Y1 purinergic receptor, which appears to be involved in astrocyte hyperactivation. Meanwhile, the related adenosine A2A receptor may impair memory, as knocking it out improved the performance of both wild-type and AD transgenic mice in behavioral tasks.
Purinergic receptors sit on the plasma membrane and are activated by ATP or its breakdown products ADP, AMP, and adenosine. In addition to playing a role in cell metabolism, these molecules signal stress or “danger” among brain cells. The receptors comprise two main classes, P1 and P2. P1 are G-protein-coupled receptors activated by adenosine. They include the A2A subtype. P2 receptors are activated by ATP or ADP, and can be either ionotropic, in which case they are called P2X, or G-protein-coupled, called P2Y. The P2Y1 subtype signals the endoplasmic reticulum to release calcium. P2Y1 and A2A normally exist at low levels on astrocytes, but in neurodegenerative disease, they appear to run amok.
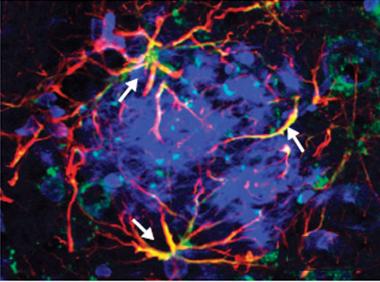
Unusual expression:
Reactive astrocytes (red) strongly express P2Y1 receptors (green, overlap in yellow) around Aβ plaques (blue). [Image courtesy of Delekate et al., Nature Communications.]
Early in AD, astrocytes in the vicinity of amyloid plaques exhibit a higher degree of spontaneous calcium activation than usual (see Kuchibhotla et al., 2009). Andrea Delekate, working with Gabor Petzold and colleagues at the German Center for Neurodegenerative Diseases (DZNE) in Bonn, went searching for the cause of this hyperactivity. Delekate implanted a cranial window in anaesthetized APPPS1 mice and monitored calcium activity in astrocytes with multiphoton microscopy. She first confirmed that the astrocytes were hyperactive, then tested whether these star-shaped cells were simply responding to neuronal activity by blocking either neuronal transmission or the astrocyte metabotropic glutamate receptors that sense it. Neither calmed the astrocytes.
Next Delekate focused on ATP receptors. In response to inflammatory signals, astrocytes, and other cells release ATP to alert surrounding neurons and glia. Previous research suggests that cells differentially express these receptors in Alzheimer’s disease, depending on the receptor subtype (for a review, see Franke et al., 2012). Could one of them explain the calcium hyperactivity? Using specific inhibitors for each purinergic receptor subtype, Delekate found that blocking P2Y1 returned astrocyte signaling to normal in the APPPS1 mice. She also confirmed previous evidence that astrocytes express unusually high amounts of P2Y1 around plaques (see image above and Moore et al., 2000). In wild-type mice, the P2Y1 inhibitor left normal astrocyte function unchanged. Delekate reported these findings, with co-first author Martina Füchtemeier, in the November 19 Nature Communications (see Delekate et al., 2014).
The researchers are now testing whether infusing the P2Y1 inhibitor into the ventricles of the brain in APP/PS1 mice for several weeks returns astrocyte activity to normal. Greg Cole, University of California, Los Angeles, questioned whether reducing astrocyte hyperactivity would be useful, since the activated astrocytes around plaques may be there to remove Aβ. “We don’t know yet if it is a good or bad thing,” said Delekate. She will explore that in treatment studies targeting different age points and disease stages to see if blocking the receptor affects memory function and disease progression.
Purines Purge Memories?
Anna Orr, who works in the lab of Lennart Mucke, Gladstone Institute of Neurological Disease, San Francisco, claimed that astrocytes in AD mouse models also overproduce the A2A receptor. Expressed by neurons and astroglia, the A2A receptor couples to Gs proteins that raise the levels of the second messenger cAMP in the cytosol. Previous studies suggested that A2A renders neurons more susceptibility to AD pathology, and that A2A antagonists prevented Aβ-induced cognitive deficits in mouse models of the disease (see Rahman, 2009, and Dall'Igna et al., 2007). However, scientists know little about how A2A expression changes in Alzheimer’s.
Orr and colleagues expected to find A2A in microglia, because they had previously found that these cells ramped up its expression upon inflammation (see Orr et al., 2009). To their surprise, they found that astrocytes, not microglia, tested positive for A2A in postmortem tissue from people with AD. The hippocampus expressed the receptor most strongly, and levels correlated positively with Braak staging—the more advanced the disease, the more A2A was expressed. Similarly, they found A2A to be up in several transgenic AD mouse lines, where the receptor accumulated once plaques started to form. The group wondered if this overexpression might damage learning and memory.
To find out, Orr and colleagues conditionally knocked out one or both copies of the gene for the A2A in the astrocytes of wild-type mice, leaving their neuronal A2A intact. These mice performed as well as controls when learning to navigate the Morris water maze, but better remembered the platform location several days later. In the same way, removing astrocytic A2A receptors in J20 mice that expressed human mutated APP improved their memory performance. Only old mice appeared to benefit from the lack of A2A—young knockouts performed no better than control wild-type mice.
In a different set of experiments, Orr elevated astrocyte Gs-coupled signaling by expressing a mutated serotonin receptor in the astrocytes of adult mice. The receptor has been engineered to bind only a synthetic ligand, and adding this agonist just before or after training the mice in the Morris water maze impaired their memories a few days later. “Together, the results imply that increases in astrocytic A2A with age are contributing to memory loss, and removing that A2A is beneficial,” Orr said.
The same could be true for people, she suggested. When given the A2A receptor blocker caffeine, people better consolidated long-term memories in a discrimination task (see Borota et al., 2014). Orr and others in the Mucke lab are now looking into what might cause A2A to be overexpressed, and what downstream mechanisms of the receptor cause these memory changes.
“The data are really interesting as they reinforce the idea that A2A receptor dysregulation may drive pathogenic mechanisms involved in AD,” David Blum, INSERM, Lille, France, wrote to Alzforum. He noted that previous studies have reported neuronal increases in A2A in the hippocampus of aging rats (see Canas et al., 2009). In addition, evidence suggests that blocking A2A protects synapses from Aβ (Canas et al., 2009) and tau (Laurent et al., 2014). “This work adds another piece to the A2A puzzle and argues for testing A2A receptor antagonists currently in trials for Parkinson’s disease in AD,” Blum wrote. A2A antagonists have shown some minor benefits for people with Parkinson’s disease as an adjunct therapy to dopaminergic drugs (see Jenner et al., 2014). One, istradefylline, has been extensively studied in Parkinson’s but was not approved by the FDA.—Gwyneth Dickey Zakaib
References
Research Models Citations
Paper Citations
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009 Feb 27;323(5918):1211-5. PubMed.
- Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012 Sep;8(3):629-57. Epub 2012 May 1 PubMed.
- Moore D, Iritani S, Chambers J, Emson P. Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer's disease. Neuroreport. 2000 Nov 27;11(17):3799-803. PubMed.
- Delekate A, Füchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun. 2014 Nov 19;5:5422. PubMed.
- Rahman A. The role of adenosine in Alzheimer's disease. Curr Neuropharmacol. 2009 Sep;7(3):207-16. PubMed.
- Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol. 2007 Jan;203(1):241-5. PubMed.
- Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. 2009 Jul;12(7):872-8. Epub 2009 Jun 14 PubMed.
- Borota D, Murray E, Keceli G, Chang A, Watabe JM, Ly M, Toscano JP, Yassa MA. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014 Feb;17(2):201-3. Epub 2014 Jan 12 PubMed.
- Canas PM, Duarte JM, Rodrigues RJ, Köfalvi A, Cunha RA. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol Aging. 2009 Nov;30(11):1877-84. Epub 2008 Mar 4 PubMed.
- Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009 Nov 25;29(47):14741-51. PubMed.
- Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y, Malik E, Mariciniak E, Parrot S, Van der Jeugd A, Faivre E, Flaten V, Ledent C, D'Hooge R, Sergeant N, Hamdane M, Humez S, Müller CE, Lopes LV, Buée L, Blum D. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol Psychiatry. 2014 Dec 2; PubMed.
- Jenner P. An overview of adenosine A2A receptor antagonists in Parkinson's disease. Int Rev Neurobiol. 2014;119:71-86. PubMed.
External Citations
Further Reading
Papers
- Rahman A. The role of adenosine in Alzheimer's disease. Curr Neuropharmacol. 2009 Sep;7(3):207-16. PubMed.
- Koutroumani M, Daniilidou M, Giannakouros T, Proitsi P, Liapi D, Germanou A, Nikolakaki E, Tsolaki M. The deletion variant of α2b-adrenergic receptor is associated with decreased risk in Alzheimer's disease and mild cognitive impairment. J Neurol Sci. 2013 May 15;328(1-2):19-23. PubMed.
- Haase N, Herse F, Spallek B, Haase H, Morano I, Qadri F, Szijártó IA, Rohm I, Yilmaz A, Warrington JP, Ryan MJ, Gollasch M, Müller DN, Dechend R, Wallukat G. Amyloid-β peptides activate α1-adrenergic cardiovascular receptors. Hypertension. 2013 Nov;62(5):966-72. Epub 2013 Sep 3 PubMed.
- Espinosa J, Rocha A, Nunes F, Costa MS, Schein V, Kazlauckas V, Kalinine E, Souza DO, Cunha RA, Porciúncula LO. Caffeine Consumption Prevents Memory Impairment, Neuronal Damage, and Adenosine A2A Receptors Upregulation in the Hippocampus of a Rat Model of Sporadic Dementia. J Alzheimers Dis. 2013 Jan 1;34(2):509-18. PubMed.
- Franke H, Illes P. Pathological potential of astroglial purinergic receptors. Adv Neurobiol. 2014;11:213-56. PubMed.
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer's disease and dementia with Lewy bodies. J Neurosci. 2006 Jan 11;26(2):467-78. PubMed.
- Haase N, Herse F, Spallek B, Haase H, Morano I, Qadri F, Szijártó IA, Rohm I, Yilmaz A, Warrington JP, Ryan MJ, Gollasch M, Müller DN, Dechend R, Wallukat G. Amyloid-β peptides activate α1-adrenergic cardiovascular receptors. Hypertension. 2013 Nov;62(5):966-72. Epub 2013 Sep 3 PubMed.
Primary Papers
- Delekate A, Füchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun. 2014 Nov 19;5:5422. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.