Dementia with Lewy Bodies: Is the Research Ready For Clinical Trials
Quick Links
Marked by degeneration of both the mind and the body, dementia with Lewy bodies (DLB) has for the past 20 years languished on the margins of medical research as an overlooked, mysterious hybrid of Alzheimer’s and Parkinson’s diseases. Largely unrecognized as a disease of its own, DLB has tended to be classified as one of its larger, eponymous cousins depending on whether a patient first sought a diagnosis for his or her symptoms from a dementia or a movement-disorder specialist—that is, if they saw a specialist at all. In fact, getting a DLB diagnosis still typically means an 18-month odyssey from doctor to doctor, and misdiagnoses along the way. However, as the International Dementia with Lewy Body Conference held December 1-4 in Fort Lauderdale, Florida, made abundantly clear, the field is shedding its relative obscurity.

In DLB, Lewy bodies (large pink sphere) form in neurons in the brain stem (top), the cortex (bottom), and other areas of the nervous system). [Courtesy of Kenji Kosaka.]
Hosted jointly by the Mayo Clinic and the Lewy Body Dementia Association, the meeting drew 400 researchers, patients, caregivers, funders and related stakeholders from across the world for a packed, 12 hour-a-day program. Attendees exchanged data in 55 talks, 117 posters, and evening symposia, while working groups of leading clinicians and scientists tried to forge consensus on how best to diagnose, manage patients, establish biomarkers, and harmonize worldwide research on DLB going forward. Patients and their care partners convened in a parallel track, but they also met with panels of clinicians and wove in and out of the scientific sessions to pepper researchers with questions.
Covering all aspects of DLB, the conference generated an overall sense that scientists have made progress in grasping this multifaceted disease. This has persuaded the pharmaceutical company Axovant to begin testing two investigational drugs specifically in DLB starting in 2016. Until recently, scientists across academia, industry and—key for drug development—at the Food and Drug Administration questioned whether DLB truly was a unique disease that needed its own indication and reimbursement code. In that regard, the psychiatric diagnostic bible, the Diagnostic and Statistical Manual of Mental Disorders, did the field a favor by adding “Mild or Major Neurocognitive Disorder with Lewy Bodies” into its disease-classification system to its current, fifth version. This gives DLB an insurance reimbursement code and helps validate its identity, said Ian McKeith of Newcastle University, U.K.
In the absence of drugs approved for DLB, practicing clinicians who recognize the disease are using various drugs indicated for AD, PD, or psychiatric and sleep conditions to manage the complex and sometimes contradictory needs of their DLB patients, said Zuzana Walker of University College, London. While allowed, pervasive off-label use creates unease among prescribing physicians, McKeith said. Clinicians need not only stronger evidence of how approved drugs perform in DLB, but also new drugs being evaluated specifically in DLB, agreed Bradley Boeve of the Mayo Clinic in Rochester, Minnesota, who co-organized the meeting. This conference series first offers a primer about DLB, and then highlights selected topics.
What, Exactly, Is DLB Again?
You might be forgiven for asking. As are AD and PD, DLB is a Lewy body disease marked primarily by pathologic aggregates of the synaptic protein α-synuclein, which are thought to spread from brain area to brain area over the course of years. However, DLB is heterogeneous in many ways. First, it is not a disease of a single neurotransmitter system, in the way Alzheimer’s is primarily seen as a cholinergic and PD as a dopaminergic disorder. Besides those two neuron types, Lewy pathology in DLB also settles in noradrenergic and serotonergic neurons, said Dennis Dickson of the Mayo Clinic in Jacksonville, Florida. In fact, the only major neuron type that seems to escape α-synuclein deposition in DLB are GABAergic ones, said Glenda Halliday of the University of New South Wales, Sydney.
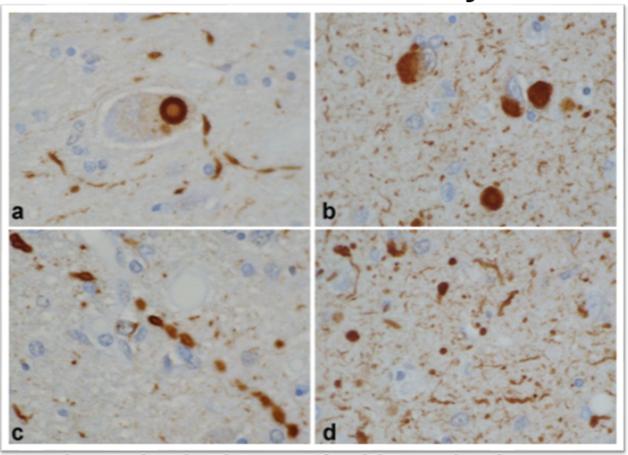
In DLB, α-synuclein pathology comes in many shapes besides the signature, large Lewy body next to the nucleus (a, b). Small Lewy bodies and other aggregates in neurites (c, d) are even more abundant. [Courtesy of Dennis Dickson, Mayo Clinic.]
Second, DLB is not a focal disease. It attacks areas across the brain, from the olfactory bulb in the front to the visual cortex in the back, from the neocortex on top to the amygdala and brain stem deep within. DLB isn’t just a brain disease, either. Branches of the autonomic nervous system innervating the heart, the intestine, the bladder, and even sex organs also degenerate, slowly erasing the function of the central and peripheral nervous system.
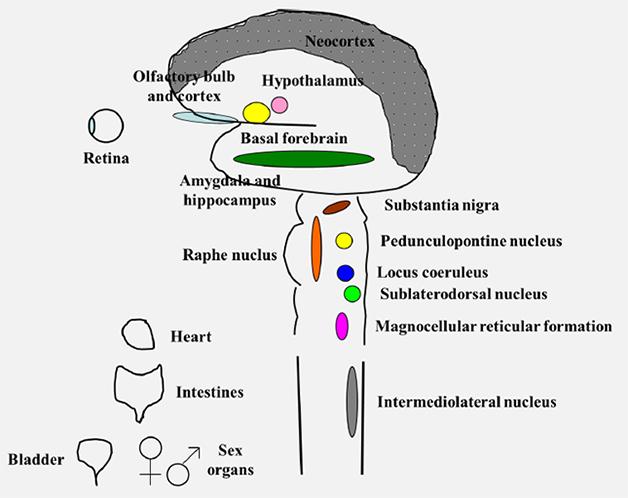
DLB attacks many parts of the central and peripheral nervous system. [Courtesy of Bradley Boeve.]
This leaves patients and caregivers to grapple with a forever-growing list of symptoms in six different functional domains, and with frequent health crises and trips to the emergency room where medical staff are sometimes unaware of the full spectrum of DLB symptoms.
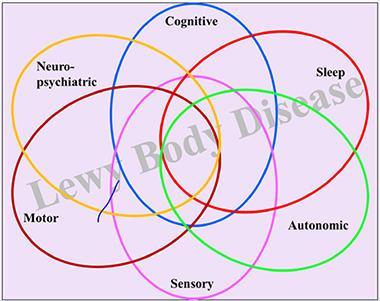
DLB’s overlapping symptoms fall into six functional domains. [Courtesy of Bradley Boeve.]
Third, DLB is heterogeneous in the sense that not every patient has the same, or all, DLB symptoms. Some suffer most from decline in the cognitive (i.e., impairment, fluctuating attention, dementia) and motor domains (i.e., the slow and stooped, shuffling walk of parkinsonism). Others are tortured by neuropsychiatric demons (visual hallucinations, delusions, depression, and anxiety), and/or suffer from urinary incontinence, constipation, and erectile dysfunction. Some decline rapidly in the face of orthostatic hypotension that starves the brain of blood and renders them prone to falls. Many DLB patients have lost their sense of smell and some see colors differently. Almost all DLB patients have sleep problems. They act out dreams at night, sometimes injuring their bed partner, are sleepless at night, and excessively drowsy during the day. “I have not met a DLB patient who does not have some sleep disorder,” said Boeve. Sleep is regulated in parts of in the brain stem and hypothalamus, regions riddled with α-synuclein pathology early on in PD and many instances of DLB.
The type of dementia in DLB differs from that of AD in that language and memory often remain relatively intact but attention, executive, and visuospatial function get worse. An engineer will lose his facility with home electronics, a planner her ability to coordinate events.
Many of these symptoms can occur in Alzheimer’s or Parkinson’s or indeed some psychiatric conditions, as well, and this overlap is one reason why many DLB cases are incorrectly diagnosed. To lay down a DLB pattern, clinicians have appointed dementia, cognitive fluctuations, visual hallucinations, parkinsonism, and REM sleep behavior disorder (RBD) as core features that distinguish DLB from other conditions. Consensus criteria published 10 years ago organize the list of symptoms into a diagnostic algorithm in an effort to capture as many different symptom combinations a given patient has at the time of diagnosis (McKeith et al., 2005). These criteria have worked reasonably well overall even though many cases are still missed, Boeve and Dickson said. In the wake of the Fort Lauderdale conference, they will be updated to include new insights, likely giving more prominence to sleep disturbances and cardiac diagnostic imaging.
DLB Research—A Thumbnail History
Research in this young field started in Japan and the United Kingdom. In Japan, Kenji Kosaka at Yokohama City University School of Medicine first connected Lewy bodies—which were thought to be the province of Parkinson’s alone—with dementia. Kosaka had been reporting in the ’70s what he then thought were isolated cases of people whose disease started with tremors and forgetfulness and progressed to dementia and parkinsonism, and who had both cortical Lewy bodies and AD pathology in their brain, (e.g., Kosaka et al., 1976).

This drawing by Kenji Kosaka details the distribution of Lewy pathology in a 65-year-old woman, his first case published in 1976. [Courtesy of Kenji Kosaka.]
Kosaka subsequently published the first European DLB cases while on a sabbatical in Munich in 1979. In 1980, back home, he proposed the term Lewy body disease along with three flavors: “brain stem” (what clinically shows up as PD), “transitional,” and “diffuse.” The latter two present clinically as DLB, and have more broadly distributed α-synuclein deposits than the former.
Two oceans away, in Newcastle, McKeith started realizing in the early 1980s that some presumed Alzheimer’s cases must have something else. In Fort Lauderdale, he recalled a 70-year-old man with an expressionless face who was moody, apathetic, and sleepy, then started seeing soldiers in a tree outside his house, walked with a stooped gait, and had trouble copying simple drawings. He deteriorated rapidly, indeed fatally, on haloperidol, and his brain proved to have been full of not only AD pathology but also Lewy bodies.
In the 1990s, Kosaka reported that DLB most commonly occurs together with Alzheimer’s plaques and tangles and more rarely as a pure α-synuclein pathology. McKeith realized that a clinical phenomenon was emerging internationally but everyone was calling it different terms, so in 1995 he invited Kosaka and other leading clinicians to gather for a first International Workshop on Dementia with Lewy Bodies in Newcastle. Numbering 170 at the time, the budding DLB community formed a consortium. They hammered out initial consensus criteria to diagnose DLB, published them together (McKeith et al., 1996), and met again three years later at the International Conference on Alzheimer’s Disease in Amsterdam to interest the broader world community of dementia researchers in DLB. At this point the DLB consortium decided that depression and sleep disorders belonged in the evolving definition of the disease, as well.
In 2003, the DLB pioneers met again and improved the diagnosis and management criteria (McKeith et al., 2005). By providing a consistent and stable classification and terminology, this so-called “Third Report” has since become the touchstone of DLB research.
In 2006, a meeting in Yokohama introduced scintigraphy imaging with the tracer MIBG. Kosaka subsequently founded a national society for DLB research, which every November convenes Japanese DLB scientists and families to drive the research agenda forward. This engaged clinicians throughout the country, who then ran clinical trials and accomplished registration of donepezil for DLB in Japan.
DLB researchers met again for a few smaller meetings, for example at the 2006 World Parkinson’s Congress in Washington, D.C. (Lippa et al., 2007), at a dedicated DLB workshop organized by Brit Mollenhauer in Kassel, Germany, in 2009, and for subsequent sessions at AD/PD and elsewhere (see May 2009 conference news; Jun 2009 conference news). Except in Japan, however, interest in DLB on the part of funders, as well as the field’s publication output, flagged for a while until there was a renewed upsurge in the past year, McKeith said.
How Common is DLB?
If only researchers knew. One problem, said McKeith, is that while the consensus diagnostic criteria have proven in the past decade to be quite specific—that is, they distinguish DLB correctly from its overlapping diseases—they are not sensitive. They miss DLB in many cases where it is actually present. That is in part because many diagnosing physicians in primary, secondary, and even some tertiary care settings are untrained in DLB and most commonly diagnose AD or PD, or a psychiatric condition, McKeith said. Epidemiology surveys thus count an unknown number of DLB as something else. Published epidemiology estimates for DLB run to about 4 percent of dementia diagnosed clinically, plus 2 percent of dementia are diagnosed with Parkinson’s disease dementia (PDD), which occurs on the same pathological spectrum. Autopsy reports flag 10 to 15 percent of lifetime dementia cases as DLB, and Kosaka told the audience in Fort Lauderdale that DLB accounts for up to 20 percent of dementia in his country. DLB is more common in men than women, and tends to become overtly symptomatic in the 70s after a long prodromal stage.
How to Detect DLB?
Beyond symptoms, physicians have some help from brain imaging in telling apart DLB and PD. For example, fitting the memory preservation in DLB, the medial temporal lobe, especially the hippocampus within it, is shrunken in AD but relatively intact in DLB. This is apparent on MRIs or even CT scans, and used in routine clinical care. In research settings, FDG PET reveals characteristically reduced metabolism in the occipital cortex of the brain, which fits with the visual impairment in DLB, as well as a curious feature called the posterior cingulate island sign. Importantly, Iodine-123 Ioflupane CIT SPECT, trade named DaTscan in Europe and the United States, visualizes loss of dopamine transporters, i.e., dopaminergic neurodegeneration, in the striatum. It is approved and widely available in Europe and Japan. In 2013, CIT SPECT also got the U.S. FDA’s blessing for differentiating tremor associated with PD from essential tremor. CIT scans well distinguish DLB from AD, though less well from related diseases on the parkinsonian side of the spectrum.
While these tools are somewhat helpful where they are available, they do not always clinch a diagnosis, said Boeve. MRI and FDG PET signals miss many cases, and clinicians need more definitive tools. While the field is eagerly awaiting a PET tracer for α-synuclein, scientists are searching for better methods or multimodal combinations of current methods. In Fort Lauderdale, researchers presented new data on both (see Part 3 and Part 4 of this series). However, the standout was the scintigraphy tracer MIBG, which in the United States is sold as AdreView but not used for DLB (see Part 2 of this series).
Fluid biomarkers for DLB are retooling in that a new set of commercial α-synuclein CSF assays are being developed at the Belgian biotech company ADx Neurosciences based on recent work by Omar El-Agnaf at Hamad Bin Khalifa University, Doha, Qatar. Proteomic or lipidomic blood-based markers are being actively pursued but remain at an earlier research stage.
Much remains to be done to improve the diagnosis of DLB. In the United States, for example, a review of a formidable clinico-pathological data set compiled by the National Alzheimer’s Coordinating Center, aka NACC, showed that only 12 to 30 percent of past DLB cases were recognized even by clinicians at the federally funded network of Alzheimer’s centers (Nelson et al., 2010). Some of these NACC data precede broad awareness of DLB as a common disease. In Fort Lauderdale, experts agreed that in 2015, diagnosis in tertiary care settings works somewhat better, and some studies to validate the 2005 diagnostic criteria suggest as much (Tiraboschi et al., 2015; Fujishishiro et al., 2008). But even today, some dead giveaways, such as cognitive fluctuations, remain easily missed in clinical assessment. In particular, primary care physicians and geriatricians, with the 10 to 15 minutes they have per patient, still need better, quicker tools to alert them that their patient might have DLB and need a detailed assessment.
To that end, James Galvin of Florida Atlantic University in Boca Raton, presented two screening tools. Called Quick Dementia Rating System (QDRS), one is a three-minute questionnaire to flag the presence of dementia and stage its severity (see Dec 2014 conference story). Called the Lewy Body Composite Score (LBCRS), the other is a three-minute questionnaire to tip off the primary care provider that Lewy body pathology, rather than AD, might be the underlying cause of the dementia. The LBCRS translates both the DLB criteria and Galvin’s experience watching his grandfather develop this disease into simple questions. In essence, the questions evoke observations his grandmother might have made caring for her husband. They ask about slow movements, excessive daytime sleepiness, acting out dreams, episodes of illogical thinking. “We are not reinventing the wheel. We just operationalized the consensus criteria in a way a caregiver could give us this information without using the clinician’s time,” Galvin said.
A caregiver fills out the questionnaires while in the waiting room, or online at home before coming in. By having this information beforehand, the health care provider can use his or her time during the visit with the patient to probe deeper, and may be more likely to order a full diagnostic workup or refer to specialty care. The screen is free for academics but requires licensing through the university for commercial providers, Galvin said.
If the Diagnosis Is so Easily Missed, Why Bother?
Getting the diagnosis exactly right is important for many reasons, researchers agree. Not least, the broad range of symptoms of DLB compared to pure AD or PD greatly complicates the physician’s ongoing work of managing a given patient’s disease. Physicians use an armamentarium of cholinesterase inhibitors, dopaminergics, glutamate antagonists, atypical antipsychotics, sedatives, antidepressants, sleep- and wake-promoting agents, and other drugs, because many of the individual symptoms of DLB are quite treatable. However, in doing so doctors and carers navigate a minefield of potential adverse effects.
For example, many people with DLB suffer from delusions and visual hallucinations that warrant treatment. The latter are particularly common and bothersome in DLB, and making them less frequent and less intense is a key goal of drug therapy, said Boeve. But DLB patients can be supremely sensitive to traditional neuroleptic drugs. Believing, erroneously, that a patient has AD, and treating him or her with an antipsychotic for delusions and hallucinations, which also occur in AD, can pitch a person with DLB into a severely rigid state, and sometimes a life-threatening condition called neuroleptic malignant syndrome can occur. In Fort Lauderdale, clinicians extensively debated the use of antipsychotic drugs for DLB, and agreed that even in the face of a pressing medical need, overall both their safety and effectiveness is questionable.
In addition, people with DLB cannot tolerate the anticholinergics often given to the elderly for urinary incontinence. Some need dopaminergic agents for their parkinsonism, but too much of those can worsen their hallucinations and autonomic symptoms.

Managing a DLB patient’s manifold symptoms is complicated, because many drugs used to treat one symptom can worsen another. [Courtesy of Bradley Boeve, Mayo Clinic.]
Sleep disturbances are more straightforward to treat, as both sleep-inducing and wakefulness medicines can be safe and effective. In Fort Lauderdale, Maria Lapid of the Mayo Clinic in Rochester reported results from a 12-week, open-label study of the wake-promoting drug armodafinil. This medication appeared to not only help patients stay awake during the day, but also lessen their visual hallucinations, agitation, and apathy. Caregivers, in turn, reported better quality of life. The Mayo researchers have been unable thus far to interest the drug maker in sponsoring a randomized controlled trial to confirm these data, Boeve said.
“Managing DLB patients is challenging. That is not a cliché. Clinically, every patient is different, and what you prioritize is different and changes over time. In the U.S. there are no FDA-approved agents, so we use everything off-label. There is little formal evidence, and no drug comes without negatives,” Boeve summed it up.
Ironically, while the acetylcholinesterase inhibitor donepezil was originally developed and licensed for Alzheimer’s, researchers in Fort Lauderdale claimed that it appears to work a little better in DLB than in AD, perhaps reflecting DLB’s significant cholinergic component. In Japan, a nationwide multicenter trial of donepezil in DLB led to its registration for DLB, the only instance worldwide of a drug licensed for DLB thus far (Mori et al., 2012; Ikeda 2013; but see also Ikeda et al., 2015). That is but a first step, however. “We really need new targets and new drugs, to treat DLB,” said Clive Ballard of King’s College, London.
How to Get More Therapy Trials Up and Running in DLB?
To plan for more treatment studies, the field is beginning to coordinate efforts around the challenge of characterizing the prodromal phase of DLB. This would help identify and enroll early stage patients into trials, much as is being done in Alzheimer’s and Parkinson’s research.
The DLB field does not yet have the biomarker staging diagrams along a time axis of progression from prodromal to MCI to the full-blown symptomatic stages that have become a staple of AD research. But the necessary pieces are moving into place. Longitudinal MCI studies have exposed a helpful dichotomy whereby amnestic MCI tends to progress to AD and non-amnestic MCI to DLB (e.g., Ferman et al., 2013). The number of studies trying to pinpoint even earlier symptoms is rapidly increasing. Observational studies to characterize people with REM sleep behavior disorder have shown that RBD usually signals underlying α-synuclein pathology and a high risk of developing DLB or PD. Alex Iranzo of the Hospital Clinic in Barcelona, Spain, mentioned that in a study of 167 RBD patients, 91 percent developed a neurodegenerative disease within 14 years (e.g., Iranzo et al., 2014).
Other studies suggest that combinations of pre-clinical features can be found to predict which α-synuclein diseases lie in a given person’s future. Daphné Génier Marchand of McGill University, Montreal, reported that in her center’s prospective cohort of 76 people with RBD followed for 10 years, cognitive tests of executive and visuospatial function versus memory can distinguish for the purpose of clinical trial enrollment which disease a person is likely to develop. Geneticists are looking for predictive variants in this cohort, as well (see Part 6 of this series). David Salmon of the University of California, San Diego, presented new cognitive tests that tax visual processing functions that are specifically impaired in DLB and help distinguish this disease from AD (e.g., Landy et al., 2015; Landy et al., 2015).
At this early stage of research, scientists are—crudely and tentatively, as they are the first to admit—marking the time axis in this proposed order. They place RBD, depression, loss of smell and/or constipation into a first, prodromal stage. Then they add in subtle cognitive problem-solving and, for some people, parkinsonian changes into an MCI stage. They place frequent visual hallucinations and fluctuations well into the symptomatic phase. The problem here, again, is that one patient may progress quite differently than another.
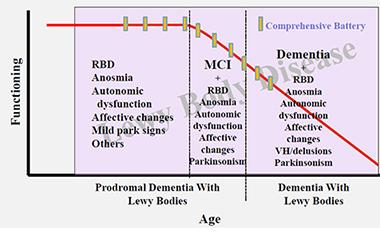
In their effort to start planning for disease-modifying trials in DLB, clinician-researchers are beginning to organize data from prodromal DLB research into a draft-staging diagram. [Courtesy of Bradley Boeve, Mayo Clinic.]
To supply this proposed staging scheme with stronger empirical data, longitudinal clinical and biomarker cohorts are beginning to form. For example, the EU-funded European DLB study (E-DLB) is currently collecting existing data on 1,208 DLB patients from more than 20 centers in 13 countries. E-DLB has published a harmonized protocol and begun enrolling for a prospective cohort, but the latter effort is not yet fully funded, said Dag Åarsland of Karolinska Institute in Stockholm. Smaller local studies are ongoing, for example the LewyPro study in Newcastle. In the United States, DLB patients tend to be followed within the federally funded ADRC program and the associated NACC database, said Thomas Montine of the University of Washington, Seattle. A national cohort study dedicated to DLB has not been launched.
In Fort Lauderdale, leading researchers discussed how to harmonize assessments and share data to enable trans-Atlantic cohorts to coalesce and gain statistical power. They agree that this will prepare the field for trials of disease-modifying drugs. At the same time, some scientists who are dissecting the relative contributions of amyloid, tau, and α-synuclein pathology to the head full of protein deposits that plague DLB patients argued that some of these patients could be made eligible for trials of anti-amyloid or anti-α-synuclein therapies even now, before the proposed trial-ready DLB cohorts are established. —Gabrielle Strobel
References
News Citations
- Spectrum of Neurodegeneration Comes to the Fore
- Neither Fish Nor Fowl—Dementia With Lewy Bodies Often Missed
- Dementia with Lewy Bodies: Sharper Image for a Formerly Blurry Disease
- Brain Imaging: What Does it See in DLB?
- Try This at Home: Cognitive Testing in the Age of Prevention Trials?
- Genetics of DLB: Setting Up to Fill a Mostly Empty Canvas
Paper Citations
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005 Dec 27;65(12):1863-72. PubMed.
- Kosaka K, Oyanagi S, Matsushita M, Hori A. Presenile dementia with Alzheimer-, Pick- and Lewy-body changes. Acta Neuropathol. 1976 Nov 15;36(3):221-33. PubMed.
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996 Nov;47(5):1113-24. PubMed.
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK, DLB/PDD Working Group. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007 Mar 13;68(11):812-9. PubMed.
- Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, Xu LO, Smith CD, Markesbery WR. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010 Mar;257(3):359-66. Epub 2009 Oct 1 PubMed.
- Tiraboschi P, Attems J, Thomas A, Brown A, Jaros E, Lett DJ, Ossola M, Perry RH, Ramsay L, Walker L, McKeith IG. Clinicians' ability to diagnose dementia with Lewy bodies is not affected by β-amyloid load. Neurology. 2015 Feb 3;84(5):496-9. Epub 2014 Dec 31 PubMed.
- Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol. 2008 Jul;67(7):649-56. PubMed.
- Mori E, Ikeda M, Kosaka K, Donepezil-DLB Study Investigators. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012 Jul;72(1):41-52. PubMed.
- Ikeda M, Mori E, Kosaka K, Iseki E, Hashimoto M, Matsukawa N, Matsuo K, Nakagawa M, Donepezil-DLB Study Investigators. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: results from a 52-week, open-label, multicenter extension study. Dement Geriatr Cogn Disord. 2013;36(3-4):229-41. Epub 2013 Aug 15 PubMed.
- Ikeda M, Mori E, Matsuo K, Nakagawa M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimers Res Ther. 2015;7(1):4. Epub 2015 Feb 3 PubMed.
- Ferman TJ, Smith GE, Kantarci K, Boeve BF, Pankratz VS, Dickson DW, Graff-Radford NR, Wszolek Z, Van Gerpen J, Uitti R, Pedraza O, Murray ME, Aakre J, Parisi J, Knopman DS, Petersen RC. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013 Dec 3;81(23):2032-8. Epub 2013 Nov 8 PubMed.
- Iranzo A, Fernández-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, Gelpi E, Vilaseca I, Sánchez-Valle R, Lladó A, Gaig C, Santamaría J. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9(2):e89741. Epub 2014 Feb 26 PubMed.
- Landy KM, Salmon DP, Filoteo JV, Heindel WC, Galasko D, Hamilton JM. Visual search in Dementia with Lewy Bodies and Alzheimer's disease. Cortex. 2015 Dec;73:228-39. Epub 2015 Sep 21 PubMed.
- Landy KM, Salmon DP, Galasko D, Filoteo JV, Festa EK, Heindel WC, Hansen LA, Hamilton JM. Motion discrimination in dementia with Lewy bodies and Alzheimer disease. Neurology. 2015 Oct 20;85(16):1376-82. Epub 2015 Sep 23 PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Cleveland Clinic
This is an excellent summary to an already successful meeting. Kudos to Alzforum for showing us the art of writing a powerful summary.
Make a Comment
To make a comment you must login or register.