At CTAD, Tau PET Emerges as Favored Outcome Biomarker for Trials
Quick Links
At the Clinical Trials on Alzheimer’s Disease meeting held November 1–4 in Boston, tau PET emerged as the crowd favorite among candidate biomarkers for measuring progression, in both natural history studies and future trials. It’s no news that tau PET tracks neurodegeneration and cognitive decline in AD better than amyloid PET. Now, researchers are pinning down details of how the tau signal changes over time and how that change relates to symptoms. These are two critical bits of information for gauging the method’s usefulness as a biomarker for prevention and treatment trials. A new PET ligand, PI-2620, offers an improved alternative to the first-generation tracer flortaucipir. With low background, PI-2620 binds multiple tau isoforms, bringing PET to non-AD tauopathies. Separately, an attempt to visualize a different aspect of Alzheimer’s—a ligand that directly measures synapses—showed losses in the hippocampus that appeared to correlate with cognitive trouble.
- Tau PET signal grows rapidly as disease progress tracks with cognitive decline.
- New tau ligand PI-2620 boasts low background, high binding to multiple isoforms.
- PET imaging of SV2A protein promises a direct measure of synaptic loss in AD.
To relate tau PET to disease progression, Bernard Hanseeuw of Harvard Medical School and Massachusetts General Hospital, Boston, has been studying participants in the Harvard Aging Brain Study (HABS) over time. At CTAD, he showed results on 60 people, median age 75. All were cognitively normal when they received baseline PiB and flortaucipir scans for amyloid and tau. They had two more scans after one and two years, and cognitive testing out to three years thus far. Hanseeuw analyzed changes in tau in their temporal neocortices.
In this group, Hanseeuw found that tau increased faster than amyloid. It would take 20 years for amyloid deposition to go up by one standard deviation, but only five years for tau. Tau PET was also the more consistent measure, showing less variability from person to person than amyloid. Both amyloid and tau deposition accelerated with time, such that the annual increase was greater in people who started out higher at baseline.
How did that relate to cognitive decline? How fast tau accumulates best predicted cognitive decline. This measure outperformed PiB accumulation, baseline PiB, or baseline tau. Even in people with high baseline amyloid and tau, cognitive decline correlated better with tau than amyloid. And while baseline PiB or tau themselves indicated future cognitive decline, the rate of tau accumulation was the stronger predictor. The results need to be validated in a larger sample with longer follow-up, Hanseeuw said. Even so, he concludes that tau PET “looks like a promising biomarker for monitoring disease progression and drug efficacy in AD trials.”
Echoing the HABS findings, Reisa Sperling, Brigham and Women’s Hospital, Boston, reported that tau is a sensitive marker for preclinical cognitive decline in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s trial cohort. A4 participants are cognitively normal with elevated amyloid. Baseline flortaucipir PET revealed that about half started the study with increased tau. The higher a person’s amyloid, the more tau they have in the medial and inferior temporal lobes and also in the posterior cingulate and precuneus areas. Sperling said for a cognitively normal group, this was striking. “In A4, we restricted the inclusion criteria to a narrow cognitive range Participants had to be normal on the MMSE, within one standard deviation of normal on other tests, and could not be supernormal. Even within this narrow band, the people with highest amyloid have more tau, and those with the highest tau have the lowest memory scores,” Sperling said.
“These data really highlight that tau PET imaging is a main outcome measure we should consider in clinical trials,” said Oskar Hansson of Sweden’s Lund University. Hansson had previously followed observational cohorts with fluid biomarkers for many years, but at CTAD he offered more validation of flortaucipir in AD from the Swedish BioFinder and other studies. Hansson compared flortaucipir PET with cerebrospinal fluid tau. CSF tau increases in late preclinical stages, when people are still cognitively normal, but then shows little further change as symptoms progress. Hansson said tau PET correlates only moderately with CSF tau, but tracks both brain atrophy and cognitive symptoms well.
Hansson calls CSF tau a “state” marker, which signals that someone is developing AD, while tau PET serves as a staging marker, which gives information about how far along they are.
On the down side, flortaucipir has performed poorly in non-AD tauopathies. The ligand binds only weakly to four-repeat (4R) tau isoforms, like those that accumulate in corticobasal syndrome (CBS) or progressive supranuclear palsy (PSP). In Hansson’s recent work, flortaucipir was able to identify pathology in the correct brain regions in CBS, but the signal was much lower than in AD (Smith et al., 2017). In PSP, tau neuropathology correlated with FDG but not tau PET (Smith et al., 2017). Overall, Hansson concluded that flortaucipir might be good enough to identify pathology in CBD, but not PSP.

Tracking Tau. PI-2620 in five people who are positive for amyloid deposition measured by florbetaben. Tau PET signal strength reaches up to 3.5 (see scale on right) in some areas; the person on the far right was borderline amyloid-positive. [Courtesy of Andrew Stephens.]
Beyond Flortaucipir, What Else Is There?
In part because flortaucipir is not widely available, or is too expensive for some groups, at least five new tau tracers have come into development (Apr 2017 conference news). At CTAD, Andrew Stephens, Piramal Imaging, Berlin, showed off his company’s entry, PI-2620. The compound binds both the three-repeat (3R) and 4R tau isoforms, and recognizes neurofibrillary pathology in AD, Pick’s disease, and PSP. Stephens showed robust uptake of PI-2620 in a patient with PSP, with binding to the bilateral globus pallidus and substantia nigra. Quantitating the PET signal in cortical and subcortical regions clearly distinguished PSP from AD. “This is a good opportunity for us,” Stephens said.
PI-2620 rapidly enters the brain, peaks at 60 to 90 minutes, and leaves the brain. After dosing, PI-2620 completely clears the body without producing lipophilic metabolites that re-enter the brain and muddy the measurement, an occasional problem in PET tracer development. Its effective dose is comparable to that of other tracers, Stephens said, allowing for two to three scans per year while staying within radiation exposure limits. Stephens emphasized very low off-target binding, with no signal in the choroid plexus, striatum, amygdala, and other midbrain structures where other tracers have run into trouble. “Choroid plexus binding masks hippocampal signal, so getting rid of that is a significant advantage,” Stephens said. He sees no uptake in elderly controls, but sees a typical distribution, distinct from amyloid, in AD patients. Regional standardized uptake volume (SUVr) values in abnormal regions reached 3.5—a stronger signal than some other tau tracers. PI-2620’s test-retest reproducibility was good in healthy controls and AD, he said.
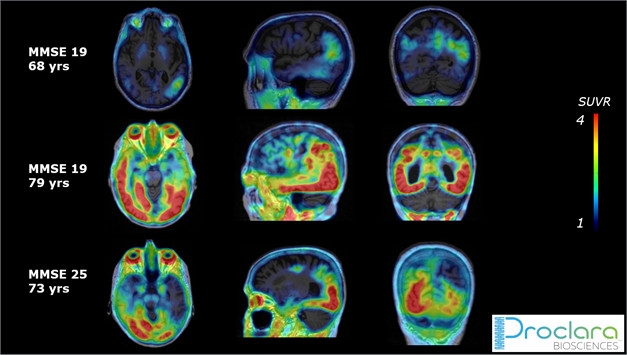
Trial Ready? Piramal’s PI-2620 shows a typical tau distribution pattern with varying degrees of accumulation in three amyloid-positive people with probable AD enrolled in the Proclara NPT088 trial. [Courtesy of Andrew Stephens.]
PI-2620 will be tested in several clinical trials, Stephens said at CTAD. The scans will serve as exploratory endpoints in a Phase 1b trial of NPT088, an investigational drug from Proclara Biosciences, Cambridge, Massachusetts. A fusion between human immunoglobulin and a bacteriophage capsid protein, the drug is a second-generation version of a prior bacteriophage therapy known to dissociate amyloid aggregates (Levenson et al., 2016; Jan 2013 conference news). In three trial participants scanned thus far with MMSEs between 25 and 19, baseline PI-2620 uptake ranged from minimal to dramatic. Stephens declined to say which other therapy trials are using PI-2620.
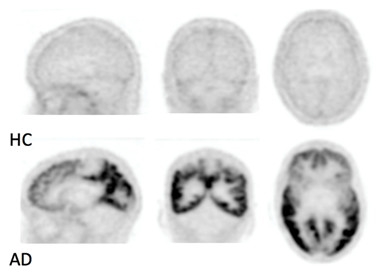
Tau en le Cervau.
In these black-and-white scans, MK-6240, a tau PET tracer developed by Merck and now Cerveau Technologies, shows no uptake in healthy controls but extensive uptake in cortical areas in a person with Alzheimer’s. [Courtesy of Christopher Rowe.]
PI-2620 is not the only new commercial tau tracer. At previous meetings, Merck researchers debuted that company’s tau PET ligand, MK-6240, which is progressing through Phase 1. Because Merck develops PET tracers for in-house use but is not a radiopharmaceuticals company, in January 2017 Merck licensed the probe to Cerveau Technologies, a partnership between Enigma Biomedical Group of Toronto and Sinotau Pharmaceutical Group in Beijing. Backed by a consortium of pharmaceutical companies, Cerveau is managing the development of MK-6240 and establishing distribution centers worldwide so it can be used in clinical and academic trials. Cerveau moved fast, and by March had secured a manufacturer and initiated research collaborations with Pedro Rosa-Neto at McGill University in Montreal, Sterling Johnson at University of Wisconsin-Madison, and Christopher Rowe at Austin Health in Melbourne, Australia, all of whom are now studying the tracer in people.
No data on MK-6240 were presented at CTAD, but just prior to the conference, Cerveau announced a partnership with the DIAN-TU at Washington University, St. Louis. Academic groups that will use the probe include Banner Health, Phoenix, New York University, the Clinical Imaging Research Center in Singapore, Massachusetts General Hospital, New York City’s Columbia University Medical Center, Houston Methodist Hospital, VU Medical Center in Amsterdam, and the Centre for Addiction and Mental Health (CAMH) in Toronto, as well as the pharmaceutical companies Biogen, AbbVie, Janssen, and Lundbeck. Rowe told Alzforum that more than 100 human studies have now been done. Thus far, the tracer shows high binding in people with AD, but no non-specific or off-target background uptake in the brain. The tracer will be in multiple clinical trials in 2018, according to Rowe.
What About Synapses—When Can We See Them?
A different substrate of Alzheimer’s disease—synapses—has traditionally lacked in vivo human biomarkers, even though researcher have long known that synaptic loss is a major structural correlate of cognitive impairment. In the CSF, this gap is starting to fill with the emergence of neurogranin, and PET may be next.

Synaptic Snapshot.
11C-UCB-J reveals reduced SV2A binding in a patient with AD (right) compared to an older control (left). [Courtesy of Christopher van Dyck.]
Historically, glucose metabolism, measured by FDG-PET, has provided an indirect measure of neuronal activity. Now, a new PET ligand for the synaptic vesicle glycoprotein 2A (SV2A) promises to measure synaptic density more directly. At CTAD, Christopher van Dyck, Yale University, New Haven, Connecticut, presented initial work with 11C-UCB-J in 10 people with AD. Developed by Richard Carson at Yale and UCB Pharma in Belgium, this probe quickly enters the brain, where it binds predominantly gray matter. In people with epilepsy, the compound can detect areas of synaptic loss near the focal point of seizures (Jul 2016 news; Finnema et al., 2017).
In the new work, van Dyck, Ming-Kai Chen, and colleagues scanned 11 PiB-negative, cognitively normal controls and 10 people who were PiB-positive, including five with mild dementia and five with amnestic MCI, in a high-resolution research scanner. They focused on the medial temporal lobe. They expected that early degeneration of entorhinal cortical projecting neurons might thin out the synapses of these cells. Also, hippocampal SV2A protein reduction has been documented in postmortem AD brains (Braak et al., 2011; Robinson et al., 2014).
Indeed, participants with AD had an average 41 percent reduction in tracer binding in the hippocampus. A reference region, the centrum seminovale, did not differ between the groups. The pattern of reduction in 11C-UCB-J differed from FDG-PET, which reflects more widespread decline in synaptic activity in the temporal, parietal, and posterior cingulate regions. In this group of participants, the loss of hippocampal binding correlated with scores on episodic memory and the CDR-sum of boxes.
An exploratory whole-brain analysis in a subset of the volunteers indicated a decrease in the entorhinal cortex, though this finding disappeared upon correction for atrophy; in contrast, the hippocampal change remained robust. Van Dyck believes he will see more differences in other regions, including the association cortex, once he looks at larger sample sizes across the range of disease severity. “Our initial experience is that the medial temporal lobe changes are much bigger than anything else in early stage disease,” he said.
Going forward, van Dyck said his group will define how 11C-UCB-J relates to cognitive decline, FDG, and tau PET. They have partnered with Cognition Therapeutics Inc., Pittsburgh, to use the tracer in an upcoming trial of the anti-Aβ oligomer therapy CT1812 and see if the tracer can detect a protective effect on synapses that has been hypothesized for this drug (see Part 10 of this series). Van Dyck told Alzforum that he hopes to see data from independent groups evaluating this tracer before long.—Pat McCaffrey and Gabrielle Strobel
References
News Citations
- Next-Generation Tau PET Tracers Strut Their Stuff
- Zuers—No Pill or Drip: Scientists Inject Phage Drug Into CSF
- Next Up for Human Brain Imaging: Synaptic Density?
- Elusive or Not, Aβ Oligomers Are in BioPharma Crosshairs
Biomarker Meta Analysis Citations
Paper Citations
- Smith R, Schöll M, Widner H, van Westen D, Svenningsson P, Hägerström D, Ohlsson T, Jögi J, Nilsson C, Hansson O. In vivo retention of (18)F-AV-1451 in corticobasal syndrome. Neurology. 2017 Aug 22;89(8):845-853. Epub 2017 Jul 28 PubMed.
- Smith R, Schöll M, Honer M, Nilsson CF, Englund E, Hansson O. Tau neuropathology correlates with FDG-PET, but not AV-1451-PET, in progressive supranuclear palsy. Acta Neuropathol. 2016 Nov 29; PubMed.
- Levenson JM, Schroeter S, Carroll JC, Cullen V, Asp E, Proschitsky M, Chung CH, Gilead S, Nadeem M, Dodiya HB, Shoaga S, Mufson EJ, Tsubery H, Krishnan R, Wright J, Solomon B, Fisher R, Gannon KS. NPT088 reduces both amyloid-β and tau pathologies in transgenic mice. Alzheimers Dement (N Y). 2016 Sep;2(3):141-155. Epub 2016 Jul 14 PubMed.
- Finnema SJ, Nabulsi NB, Mercier J, Lin SF, Chen MK, Matuskey D, Gallezot JD, Henry S, Hannestad J, Huang Y, Carson RE. Kinetic evaluation and test-retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2017 Jan 1;:271678X17724947. PubMed.
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011 Nov;70(11):960-9. PubMed.
- Robinson JL, Molina-Porcel L, Corrada MM, Raible K, Lee EB, Lee VM, Kawas CH, Trojanowski JQ. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer's disease in the oldest-old. Brain. 2014 Sep;137(Pt 9):2578-87. Epub 2014 Jul 9 PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.