Pimavanserin Trial Raises Hope for Treating Dementia-Related Psychosis
Quick Links
Advancing dementia can bring with it psychosis, which greatly adds to the burden of life with Alzheimer’s disease for both the sufferer and his or her carers. No drugs are approved to treat dementia-related psychosis. Used off-label, psychiatric drugs tend to offer little benefit but much risk, from faster cognitive decline to higher mortality due to stroke and other causes.
- No drugs are approved to treat dementia-related psychosis.
- Pimavanserin reduced psychotic symptoms in people with AD.
- Unlike other antipsychotics, this drug seemed safe.
The serotonin 5-HT2A receptor antagonist pimavanserin was recently approved for treating psychosis related to Parkinson’s disease, and at the Clinical Trials on Alzheimer’s Disease conference, held November 1–4 in Boston, Clive Ballard of the University of Exeter Medical School in the U.K. told the assembled scientists that pimavanserin now looks promising for Alzheimer’s, too. In a Phase 2 study, a six-week course of pimavanserin significantly reduced psychosis in people with AD, with no significant adverse effects and no negative effect on cognitive function.
“This is a beneficial treatment. We have to do a Phase 3, but compared to very high adverse event rates with previous antipsychotics, this seems like a large step forward,” said Ballard.
Pierre Tariot, Banner Health Institute, Phoenix, concurred. “We use current antipsychotics at our peril. With them, the people who benefit without being harmed are few. I hope pimavanserin will improve this situation,” Tariot told the audience.
Developed by Acadia Pharmaceuticals, San Diego, California, pimavanserin won FDA approval in 2016 for the treatment of hallucinations and delusions associated with Parkinson’s disease (Apr 2016 news).
To test the drug in psychosis related to dementia, Ballard’s trial treated 181 Alzheimer’s patients from 133 nursing homes in the U.K. with 34 mg of pimavanserin or placebo once daily for six weeks, with an additional six-week extension. Participants were mostly women in their mid- to late 80s, with possible or probable AD and psychotic symptoms that developed after dementia started. One-third had severe psychosis, and all had at least mild symptoms every day, or severe symptoms once a week or more.
The trial’s primary endpoint was the NPI-NH psychosis score at six weeks, and secondary endpoints measured global cognition, agitation, and safety. Ballard said he included broad assessments such the ADCS-Clinical Global Impression of Change to measure general functioning that might also flag any safety issues. The analysis included patients who took at least one dose of medication and had at least one post-dose psychiatric assessment.
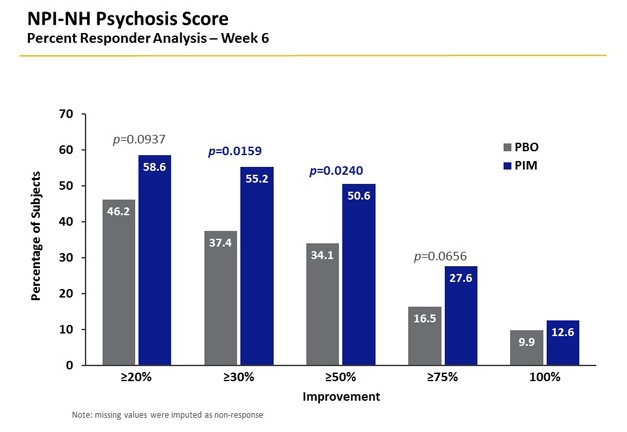
Blue is Better. Responder analysis indicates how many patients improve, and by how much, in a clinical trial. It is a measure of clinical relevance. More people on pimavanserin (blue) bettered their psychosis scores than those on placebo (gray). [Courtesy of Clive Ballard.]
Over the six-week treatment, psychosis scores declined in both pimavanserin and placebo groups, but more so in the former. This group experienced nearly 4 points of reduction in the NPI-NH score, where lower scores are better. This was 39.5 percent more than seen in the placebo group. Just over 55 percent of the participants showed a response to drug (defined as a 30 percent improvement in score), compared to 37 percent responding to placebo. Both the drop in NPI-NH score and increased response rate were statistically significant. This effect size exceeded that of previous trials of atypical antipsychotics, Ballard said.
He saw no relapse when the drug was continued for another six weeks; however, the placebo group continued to improve, such that there was no longer a difference between placebo and treated groups by 12 weeks. “The benefit is substantial, but we cannot say if it was sustained,” he said.
Stephen Salloway of Brown University, Providence, Rhode Island, questioned this timing, where the treated group did better than placebo at six but not 12 weeks. Ballard noted that the decline in NPI-NH scores in the placebo group over 12 weeks was consistent with the known remitting-recurring nature of psychotic symptoms in people with AD. Indeed, 70 percent of patients improve over 12 weeks, though half will relapse within a year. “Either pimavanserin has an acute effect that is lost later, or the symptoms are remitting-recurring,” Ballard said. “We need to do a longer trial to see if pimavanserin can prevent their recurrence.”
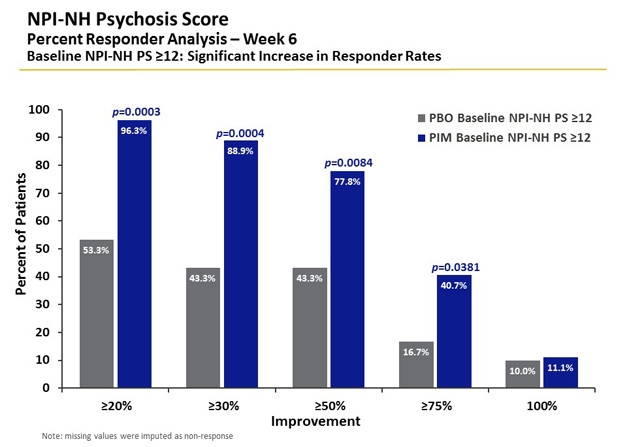
Worse Symptoms, More Relief. The majority of people with severe psychosis responded to pimavanserin (blue). Nearly 80 percent of patients showed greater than 50 percent improvement after six weeks, twice that seen in the placebo group (gray). [Courtesy of Clive Ballard.]
Patients whose symptoms were worse at baseline showed a better response, and pimavanserin reduced psychosis symptoms by 66 percent in people with severe psychosis. People who had used antipsychotics before, or whose baseline scores for agitation/aggression were high, also responded well to pimavanserin. People with concurrent severe psychosis and agitation had the greatest benefit. “Anything that’s indicating higher severity predicts a better response,” Ballard said. There was some suggestion of greater benefit with concurrent SSRI use.
The treatment raised no new safety flags over previous trials. The drug group did have more serious adverse events compared to placebo, but few people discontinued the drug. In particular, the investigators saw no increase in falls, fractures, stroke, or mortality—all concerns with atypical antipsychotics. The one new safety sign was that a small proportion of the drug-treated group lost weight, which had not been seen in previous trials. This needs to be studied further, Ballard and others at CTAD agreed.
Importantly, preliminary results suggested that treatment did not affect cognitive function, as the MMSE stayed stable in both groups over the 12-week trial.
In September, Acadia Pharmaceuticals began a six-month Phase 3 trial to evaluate pimavanserin’s ability to prevent relapse of psychosis in patients with Alzheimer’s or other dementias, including dementia with Lewy bodies, Parkinson’s disease dementia, and vascular and frontotemporal dementia. Participants whose symptoms stablilze after 12 weeks on pimavanserin will be radomized to drug or placebo, and then followed until relapse. Acadia is also running a 1-year open-label Phase 2 trial of pimavanserin in people whose AD is accompanied by agitation and aggression.
The need for new medications in dementia has only grown as nursing homes have begun to rein in excessive use of antipsychotics in these patients (Gurwitz et al., 2017).—Pat McCaffrey.
References
Therapeutics Citations
News Citations
Paper Citations
- Gurwitz JH, Bonner A, Berwick DM. Reducing Excessive Use of Antipsychotic Agents in Nursing Homes. JAMA. 2017 Jul 11;318(2):118-119. PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.