Blood Amyloid Test May Help Diagnose Alzheimer’s, but Questions Remain
Quick Links
Fluid tests for Alzheimer’s disease are hurtling toward widespread use. At the Clinical Trials on Alzheimer’s Disease conference, held November 29 through December 2 in San Francisco, several groups updated current research toward that goal. Joel Braunstein of C2N Diagnostics in St. Louis said that his company's Aβ42/40 assay, PrecivityADTM, helped dementia specialists clarify whether patients with memory problems had AD or not. Other researchers cautioned that blood tests must withstand not only the complicated milieu of plasma but also variability from frequent testing and drug interactions before they will perform robustly in routine clinical practice.
- PrecivityADTM results led clinicians to prescribe or revoke AD drugs.
- Comorbidities, common medications influence plasma Aβ levels.
- Blood tests for AD not quite ready for routine clinical use.
- This month, FDA approved Roche’s p-tau181/Aβ42 CSF test.
PrecivityADTM is being marketed next to two fully FDA-approved cerebrospinal fluid amyloid assays. One, Fujirebio’s Lumipulse Aβ42/40, received the U.S. agency's approval earlier this year. On December 8, Roche’s Elecsys CSF phospho-tau181/Aβ42 assay also got FDA’s green light to be used in clinical practice as a diagnostic aid.
C2N’s PrecivityADTM was the first AD blood test to be certified for clinical use in the U.S. under the Clinical Laboratory Improvement Amendments protocol (Nov 2020 news). The assay measures plasma Aβ42/40 and ApoE proteotype. Adding in age renders an Amyloid Probability Score (APS), a number from zero to 100 that estimates how likely the person is to have amyloid plaques. A score from zero to 35 means low probability, 36 to 57 intermediate, and 58 to 100 high. In his CTAD talk, Braunstein said the test diagnoses AD with 85 percent accuracy, but also acknowledged that, to calculate that value, C2N excluded the 14 percent of patients whose results fell into the intermediate category (see also Hu et al., 2022).
Do APS scores help practicing clinicians decide whether the patient before them has brain amyloid deposition and, therefore, help them give a more confident AD diagnosis? C2N ran a study to find out. The company asked 43 memory care specialists from 15 clinics across the U.S. how likely they thought it was that their patient had AD before and after PrecivityADTM testing. The clinicians evaluated 347 people who were 60 years or older and had signs of mild cognitive impairment or dementia. Their average age was 74 years; 90 percent were Caucasian. Of the participants, 133 had low APS scores, 52 intermediate, and 162 high.
The overall probability of an AD diagnosis in the group remained the same before and after the test, Braunstein reported. Still, these dementia specialists were more confident in diagnosing, or excluding, AD after seeing individual patient APS scores (see image below). Before testing, clinicians reported being 58 percent sure about an AD diagnosis in people who ended up having low scores; this dropped to 23 percent certainty once clinicians saw the score. Confidence for patients with intermediate scores dropped slightly, going from 64 percent to 52 percent likelihood of AD. In people with high scores, AD likelihood rose from 71 to 89 percent pre- to post-test.
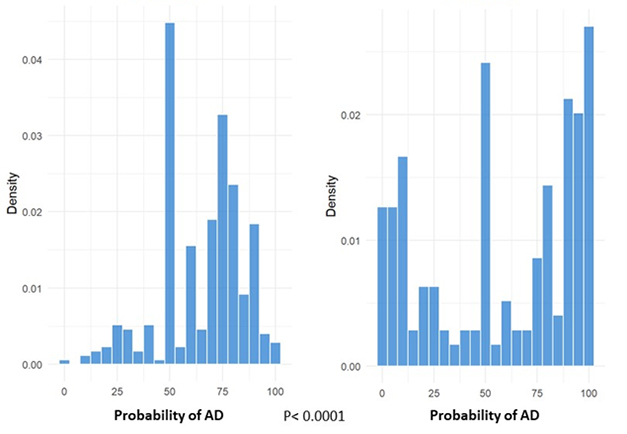
More Confidence? While dementia specialists were on the fence about whether a patient with memory troubles had AD before PrecivityADTM testing (left), they more confidently diagnosed or excluded AD after considering results of the combined blood Aβ ratio/ApoE/age algorithm (right). [Courtesy of Joel Braunstein, C2N Diagnostics.]
The dementia specialists' greater confidence—for those patients whose results fell outside the test's intermediate range—translated to changes in prescribing. They took about half of the participants with low APS scores off AD drugs, while prescriptions rose by one-third among people with high scores.
All told, Braunstein concluded that PrecivityADTM was useful to dementia specialists in deciding on an AD diagnosis in people with memory problems, which, he noted, was the test’s intended clinical usage. Braunstein said C2N plans to apply for FDA approval, but did not specify when.
Ready for Prime Time?
Some scientists question whether plasma Aβ42/40 is a sufficiently robust marker for clinical use. In his keynote, Kaj Blennow of the University of Gothenburg, Sweden, emphasized that the Aβ 42/40 ratio falls only by about 8 to 12 percent in the blood of people with plaques in their brains, because Aβ is also produced in the periphery (Janelidze et al., 2021). This is a biological, and immutable, problem with the analyte, Blennow said, not with any company's assay.
To minimize variation when measuring analytes, research studies typically collect blood samples over time, and then analyze a whole batch together. They look at the distribution of the data, which is often bimodal, allowing them to draw a cutoff to distinguish positive from negative. Routine care is different. Patient samples are analyzed the day they come in, or within the week of their visit, and are deemed positive or negative as judged by a previously determined cutoff.
Tests for routine clinical use must be able to withstand more variability and, therefore, be more robust than research-grade assays. “To give a clinically consistent patient classification, a biomarker must have a total allowable error that is substantially lower than its fold change, which is difficult to do when that change is about 10 percent,” Blennow said in his keynote. Total allowable error includes perturbations arising from sample handling, differences between reagent batches, patient comorbidities, and drug effects (Benedet et al., 2022; Rabe et al., 2022).
The much larger fold change in plasma p-tau fragments during brain amyloidosis and tauopathy may partly explain why these may end up becoming more robust markers of plaques and tangles for routine clinical use (see Part 9 of this series for more on p-tau217). In a CTAD poster, Stuart Portbury of ALZpath Bio in Carlsbad, California, saw that plasma p-tau181 and p-tau217 increased 1.8- and 4.2- fold, respectively, in people with AD. Citing a paper from Adam Boxer’s lab at the University of California, San Francisco, Blennow noted a similar spike of 2.5- to fourfold for plasma p-tau181 and p-tau217, respectively, in AD (Thijssen et al., 2021). Another form of tau, made only in the brain, jumps two- to 9.5-fold in the blood of people with AD, making this new candidate marker 78 to 99 percent accurate at distinguishing AD from related diseases (see Part 12 of this series).
While clinical research on a growing number of candidate tests is galloping along at full tilt, leading biomarker researchers agree that AD blood tests be used mainly in symptomatic people in specialized memory clinics, or in clinical trials, for the time being (Aug 2022 conference news). “Down the road, blood-based biomarkers will be used in primary care to identify individuals who are amyloid-positive,” Michelle Mielke of Wake Forest University, Winston-Salem, North Carolina, said in her presentation.
Parameters being studied to assess these biomarkers' feasibility in routine clinical use include how they behave in diverse cohorts, and how they respond to potential confounders such as concurrent illnesses and commonly used medications (Aug 2022 conference news).
For example, at CTAD, Alexa Pichet Binette of Lund University, Sweden, presented findings on comorbidities (Pichet Binette et al., 2022). In her analysis, poor kidney function, as judged by blood creatinine levels, and body mass index both affected certain plasma biomarker levels, namely glial fibrillary acidic protein and neurofilament light, more strongly than they affected others, such as p-tau217 or Aβ42/40. Overall, though, these confounding factors did not influence each marker’s ability to predict future dementia, Pichet Binette said.
Pichet Binette’s results suggest that at least some comorbidities may not need to be accounted for when interpreting biomarker levels in the clinic. Henrik Zetterberg of UGot agreed. “I think kidney disease and BMI are not a big problem for plasma tau measurement,” he told Alzforum.
To the extent comorbidities do tamper with biomarkers, Mielke suggested mitigating the problem by using ratios. For example, the Aβ42/40 ratio remains unperturbed when both individual Aβ isoforms respond to the given comorbidity in the same way. Some groups are now exploring the ratio of a given phosphorylated tau species versus its unphosphorylated counterpart to see if using a ratio would help with this biomarker, too. Alas, this method is proving difficult to reproduce for p-tau217/tau217, so its usefulness remains to be determined. “We think the tau217 measurement in this ratio is contaminated by tau made peripherally,” Zetterberg said. An alternative to p-tau ratios might be to measure only brain-derived tau isoforms (see Part 12 of this series).
Besides BMI and kidney disease, other comorbidity effects have been reported, suggesting that more research will be needed in this area before plasma AD tests can be widely used. For example, in earlier work, Blennow's group saw Aβ42/40 plummet in response to hypoxia in the days following patients’ revival from cardiac arrest. Those with worse cognition after survival had even lower blood Aβ42/40 ratios (Dec 2011 news). Chronic diseases, such as diabetes, hypertension, or brain disorders that come with cortical white-matter lesions and cerebral microbleeds, all drove up plasma, but not CSF, levels of Aβ40 and Aβ42 (Janelidze et al., 2016).
As for medications that might confound AD blood tests, Blennow at CTAD presented new data from UGot’s Wagner Brum. It showed effects on the plasma Aβ42/40 ratio by the chronic heart failure drug Entresto, which the FDA approved in 2021. This combination drug contains sacubitril, an inhibitor of the Aβ-degrading enzyme neprilysin. Brum analyzed blood samples from 93 patients who enrolled in a one-year clinical trial of Entresto after they had had a heart attack. He found the plasma Aβ42/40 ratio in the Entresto group to be lower than that of the placebo group by 31 percent—three times the drop in people with brain amyloidosis.
“This could be a major confounder for plasma Aβ42/40 measurements and may lead to misclassification,” Blennow wrote to Alzforum. The change is mainly driven by Aβ40, which doubles in the plasma. “We must carefully consider prescription drug use when analyzing blood biomarkers in the clinic,” Blennow emphasized.
CSF Further Along the Path to Routine Use
While blood tests are still wending their way through this kind of applied research, CSF Aβ assays are formally ready for prime time. Fujirebio’s Lumipulse Aβ42/40 test got FDA approval in May (May 2022 news). Earlier this month, FDA similarly greenlighted Roche’s Elecsys p-tau181/Aβ42 assay (press release).
Both immunoassays are intended for use in people 55 or older with cognitive impairment. They distinguish amyloid PET-positive from -negative people with about 90 percent accuracy. The CSF Aβ42/40 is halved in amyloid-positive versus -negative people. This larger fold change compared to that of the blood amyloid ratio partly explains the ratio's higher accuracy in the CSF.
The Lumipulse and Elecsys systems are fully automated. They are available throughout the U.S. and Europe, either directly in hospital-based labs, or through commercial labs such as LabCorp. They cost far less than a PET scan, and less than PrecivityADTM. The catch? They require a lumbar puncture, which has a long safety record but meets more resistance among physicians than a venous puncture.
How readily doctors will use the Lumipulse or Elecsys tests may depend on how quickly demand for amyloid testing will rise. This could happen soon, especially if lecanemab, and potentially donanemab, receive FDA approval next year for people with a positive amyloid test.—Chelsea Weidman Burke
as
References
News Citations
- Plasma Aβ Test Wins Approval—Are p-Tau Tests Far Behind?
- Plasma P-tau217 Picks Up Plaques, Tangles, Future Decline
- Better Diagnosis with Blood Test Detecting Only Tau Made in Brain
- Alzheimer's Blood Tests Have Arrived; Road to Broad Use Still Stretches On
- Blood Tests: Charting the Path to Primary Care
- Does Brain Hypoxia Help Kick Off Alzheimer’s Pathology?
- FDA Approves Fujirebio’s CSF Test for AD—Quest Diagnostic Offers Plasma Test
Therapeutics Citations
Paper Citations
- Hu Y, Kirmess KM, Meyer MR, Rabinovici GD, Gatsonis C, Siegel BA, Whitmer RA, Apgar C, Hanna L, Kanekiyo M, Kaplow J, Koyama A, Verbel D, Holubasch MS, Knapik SS, Connor J, Contois JH, Jackson EN, Harpstrite SE, Bateman RJ, Holtzman DM, Verghese PB, Fogelman I, Braunstein JB, Yarasheski KE, West T. Assessment of a Plasma Amyloid Probability Score to Estimate Amyloid Positron Emission Tomography Findings Among Adults With Cognitive Impairment. JAMA Netw Open. 2022 Apr 1;5(4):e228392. PubMed.
- Janelidze S, Teunissen CE, Zetterberg H, Allué JA, Sarasa L, Eichenlaub U, Bittner T, Ovod V, Verberk IM, Toba K, Nakamura A, Bateman RJ, Blennow K, Hansson O. Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021 Nov 1;78(11):1375-1382. PubMed.
- Benedet AL, Brum WS, Hansson O, Alzheimer’s Disease Neuroimaging Initiative, Karikari TK, Zimmer ER, Zetterberg H, Blennow K, Ashton NJ. The accuracy and robustness of plasma biomarker models for amyloid PET positivity. Alzheimers Res Ther. 2022 Feb 7;14(1):26. PubMed.
- Rabe C, Bittner T, Jethwa A, Suridjan I, Manuilova E, Friesenhahn M, Stomrud E, Zetterberg H, Blennow K, Hansson O, Alzheimer's Disease Neuroimaging Initiative† and the Swedish BioFINDER study. Clinical performance and robustness evaluation of plasma amyloid-β42/40 prescreening. Alzheimers Dement. 2022 Sep 23; PubMed.
- Thijssen EH, La Joie R, Strom A, Fonseca C, Iaccarino L, Wolf A, Spina S, Allen IE, Cobigo Y, Heuer H, VandeVrede L, Proctor NK, Lago AL, Baker S, Sivasankaran R, Kieloch A, Kinhikar A, Yu L, Valentin MA, Jeromin A, Zetterberg H, Hansson O, Mattsson-Carlgren N, Graham D, Blennow K, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Teunissen CE, Rabinovici GD, Rojas JC, Dage JL, Boxer AL, Advancing Research and Treatment for Frontotemporal Lobar Degeneration investigators. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021 Sep;20(9):739-752. PubMed.
- Pichet Binette A, Janelidze S, Cullen N, Dage JL, Bateman RJ, Zetterberg H, Blennow K, Stomrud E, Mattsson-Carlgren N, Hansson O. Confounding factors of Alzheimer's disease plasma biomarkers and their impact on clinical performance. Alzheimers Dement. 2022 Sep 24; PubMed.
- Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, Song L, Hanlon D, Tan Hehir CA, Baker D, Blennow K, Hansson O. Plasma β-amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016 May 31;6:26801. PubMed.
Other Citations
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.