Imaging Exposes Hugely Heterogeneous Brain Changes Among FTDs
Quick Links
Frontotemporal lobar degeneration, as its name implies, involves shrinkage of the frontal and temporal lobes of the brain. Hidden beneath this moniker is a devilish complexity and variability. In some people with FTD, the right side of the brain contracts; in others, the left. Some have more degeneration in temporal areas, while frontal areas fade in others. In some, deep subcortical structures also shrivel. In response to the rarity and heterogeneity of every aspect of FTLD, large international cohort studies have applied a barrage of neuroimaging techniques to investigate patterns of atrophy, white-matter erosion, and breakdown of neural circuitry in the brains of people with sporadic and familial forms of FTD. While this work made it clear that structural changes start years before symptoms in all FTDs, these changes and their trajectories are far from the same, depending on the offending mutation with which an individual is saddled. Partly for this reason, the initiatives’ brain imaging research has not yet come up with robust, easy-to-use neuroimaging measures. Importantly, the field also lacks molecular tracers that illuminate the identity and distribution of neuropathology in the FTD brain.
- The brain shrinks in different regions, at a different pace, in carriers of MAPT, GRN, or C9ORF72 mutations.
- In some MAPT mutation carriers, the brain’s networks change in their teenage years.
- The field still lacks robust PET tracers to track underlying neuropathology.
At the International Conference on FTD, held virtually March 3–5, and in recent publications, researchers compared their latest insights in grappling with this complexity. They described how the brain changes differently across genetic forms of the disease, and closed with the hope that their findings will guide the use of neuroimaging markers in therapeutic trials, most of which are geared toward individual mutation groups.
One of the largest longitudinal imaging efforts in familial FTD to date was conducted by the Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) and Longitudinal Evaluation of Familial Frontotemporal Dementia (LEFFTDS) studies, now united under the name ALLFTD. The observational study engages 19 centers in the United States and Canada, where participants regularly submit to neuroimaging sessions. Rather than deciding a priori which region of the brain might shrink, the researchers use serial imaging data to detect any region in a person’s brain that has shrunk between one session in the scanner and the next. Using this approach and some Bayesian statistics, Adam Staffaroni of the University of California, San Francisco, and colleagues tracked the rate of brain atrophy across the brain among 100 mutation carriers and 60 relatives who were noncarriers (Staffaroni et al., 2020). In a nutshell, each type of mutation exacted its own style of wreckage on the brain.
Using worsening scores on the CDR-NACC-FTLD (see Part 1 of this series) as a gauge of disease progression, the scientists reported that in 28 MAPT mutation carriers, the temporal lobes on both sides of the brain started gradually shrinking in the presymptomatic years. Alas, as symptoms emerged and worsened, the shrinkage revved up and engulfed the frontal lobes. For 33 GRN mutation carriers, atrophy rates in the frontal and temporal lobes held steady in the presymptomatic and mild symptomatic stages of the disease, then ramped up steeply as disease got severe. C9ORF72 carriers were markedly different. For them, frontotemporal atrophy marched onward at constant clip throughout all stages of disease; it appeared untethered to symptom severity. “Although the destiny for the brain in C9ORF72 is similar to that of other pathogenic variants, the path to this point is different, being slower and more constant over time,” the authors wrote.
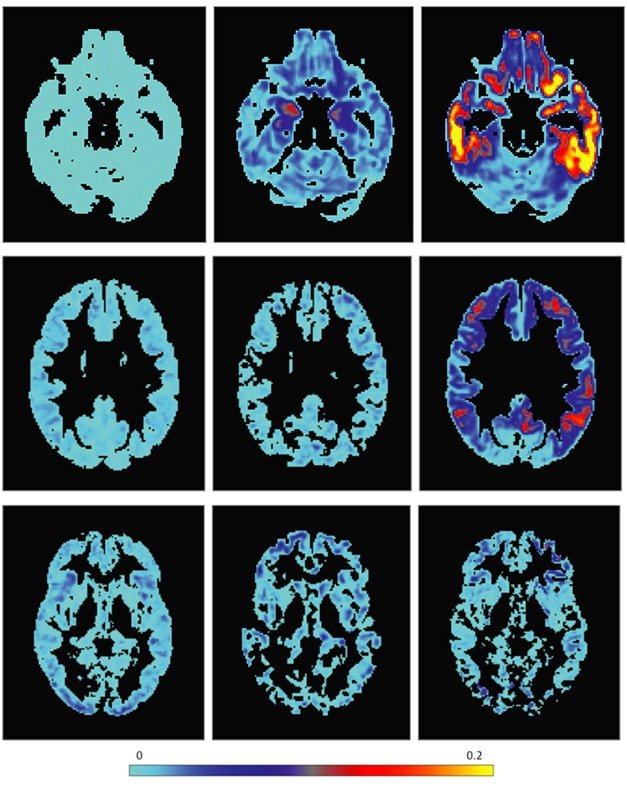
Avenues to Atrophy. Rates of atrophy across the brain, ranging from mild (light blue) to severe (yellow), were mapped for carriers of pathogenic mutations in MAPT (top), GRN (middle), and C9ORF72 (bottom), across disease stage. CDR+NACC-FTLD score of zero = presymptomatic, 0.5 = mild/questionable, 1 = symptomatic. [Courtesy of Staffaroni et al., JAMA Network Open, 2020.]
This has implications for prognosis as well as for the use of structural MRI as an outcome measure in clinical trials, the authors wrote. At ICFTD, Howard Rosen of UCSF, who led the study, discussed how unbiased imaging could be applied to track atrophy and predict symptom onset in an individual person, regardless of where degeneration started inside his or her brain. Other researchers asked how many serial measurements would be required, and how far apart they would need to be spaced, to provide useful information about progression, for example, in the context of clinical trials. Rosen said he is investigating these questions in the ALLFTD cohort.
Other neuroimaging studies have focused on one gene at a time. At ICFTD, Suzee Lee, also at UCSF, gave an update on her work with carriers of a pathogenic MAPT variant. For all symptomatic carriers, shrinkage was apparent in the frontotemporal cortex, insula, and striatum, whereas one in five presymptomatic carriers had atrophy in the medial temporal lobe, which started as early as their 30s. Lee also examined the integrity of white-matter tracts in the cohort, reporting erosion of the corpus callosum and uncinate fasciculus in symptomatic carriers, a finding that appeared among few presymptomatic carriers (Chu et al., 2021).
At ICFTD, Lee reported new findings using resting-state functional MRI. rsfMRI measures neural activity and gauges how robustly networks in the brain are functionally connected. Lee saw that the connectivity of several brain networks known to be affected in FTD was already changing in people as young as their teens. For example, in young MAPT mutation carriers, connectivity was reduced in the salience network. Dysfunction in this network is thought to underlie many of the core behavioral and emotional symptoms of FTD, including loss of empathy and awkward social interactions. Because abnormally low connectivity in this network was seen at such a young age, Lee proposed that neuronal circuitry might even develop differently in MAPT carriers. Lee can’t address the question directly because children are not participating in ALLFTD.
The new findings dovetail with Lee’s previous work describing how distinct patterns of neuronal circuitry were going awry in GRN and in C9ORF72 mutation carriers (Lee et al., 2019; Lee et al., 2017). Interestingly, while connections tended to wane in key neural networks in presymptomatic MAPT and C9ORF72 carriers, some of those same networks became hyperconnected in GRN carriers. The finding is yet another example of the bewildering variability of FTD.
Lee and other researchers are also using multimodal MRI; in other words, they are examining changes in gray-matter volume and white-matter tracts in an effort to more accurately predict symptom onset. Some studies have found that crumbling circuitry precedes gray-matter atrophy in familial FTD, predicting symptom onset within four years (Jiskoot et al., 2019; Feis et al., 2020). Alas, John van Swieten of Erasmus University in Rotterdam, who led this work, said that while shrinkage of the brain does happen in FTD’s presymptomatic phase, the profound variability in terms of which regions shrink when, and how this speeds up relative to symptoms, makes MRI less robust, and less practical, for biomarker use than fluid markers such as plasma NfL.
Ultimately, the most sensitive tools to predict symptom onset and track progression will combine fluid, neuroimaging, and cognitive measures, van Swieten believes. A recent cross-sectional GENFI analysis that modeled a cascade of biomarker changes in GRN mutation carriers exemplifies this approach (Panman et al., 2021). The study included 56 presymptomatic and 35 symptomatic GRN variant carriers, including people with PPA and bvFTD. The authors reported that language problems and plasma NfL rose first, followed by loss of integrity in specific white-matter tracts, and then gray-matter atrophy.
Curiously, for people with PPA, this sequence of changes mapped neatly onto functional and cognitive measures of disease progression, including the FTD-CDR-SOB, a modified version of the CDR-SOB that includes behavioral and functional symptoms specific to FTD. In contrast, for people with bvFTD, the biomarker cascade was only loosely tied to clinical disease severity, once again underscoring the obstreperous heterogeneity that is part and parcel of FTD.
This ICFTD was noticeably devoid of PET imaging data, which have taken Alzheimer’s research conferences by storm for many years. The FTD field still lacks molecular tracers that reliably bind to the different forms of tau that accumulate in people across the FTD spectrum, much less to the TDP-43 pathology that accumulates in about half of people with bvFTD or PPA. Some up-and-coming tracers do bind to the 4R-tauopathies in people with PSP or CBD (Jul 2020 news), but for people with sporadic FTD, the molecular identity of the neuropathology lurking in the brain cannot at present be determined during life.
Along with fluid and imaging markers, the FTD field is designing cognitive, functional, and physiological tests that measure clinical symptoms of the disease. Read the next part of the series to hear more about progress on this front.—Jessica Shugart
References
News Citations
- Merged Consortia Forge Path to Trials in Frontotemporal Dementia
- PET Tracer PI-2620 Detects 4R Tau Deposits
- Moving Target: Can Standardized Tests Track Symptoms of FTD?
Paper Citations
- Staffaroni AM, Goh SM, Cobigo Y, Ong E, Lee SE, Casaletto KB, Wolf A, Forsberg LK, Ghoshal N, Graff-Radford NR, Grossman M, Heuer HW, Hsiung GR, Kantarci K, Knopman DS, Kremers WK, Mackenzie IR, Miller BL, Pedraza O, Rascovsky K, Tartaglia MC, Wszolek ZK, Kramer JH, Kornak J, Boeve BF, Boxer AL, Rosen HJ, ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration Consortium. Rates of Brain Atrophy Across Disease Stages in Familial Frontotemporal Dementia Associated With MAPT, GRN, and C9orf72 Pathogenic Variants. JAMA Netw Open. 2020 Oct 1;3(10):e2022847. PubMed.
- Chu SA, Flagan TM, Staffaroni AM, Jiskoot LC, Deng J, Spina S, Zhang L, Sturm VE, Yokoyama JS, Seeley WW, Papma JM, Geschwind DH, Rosen HJ, Boeve BF, Boxer AL, Heuer HW, Forsberg LK, Brushaber DE, Grossman M, Coppola G, Dickerson BC, Bordelon YM, Faber K, Feldman HH, Fields JA, Fong JC, Foroud T, Gavrilova RH, Ghoshal N, Graff-Radford NR, Hsiung GR, Huey ED, Irwin DJ, Kantarci K, Kaufer DI, Karydas AM, Knopman DS, Kornak J, Kramer JH, Kukull WA, Lapid MI, Litvan I, Mackenzie IR, Mendez MF, Miller BL, Onyike CU, Pantelyat AY, Rademakers R, Marisa Ramos E, Roberson ED, Carmela Tartaglia M, Tatton NA, Toga AW, Vetor A, Weintraub S, Wong B, Wszolek ZK, ARTFL/LEFFTDS Consortium, Van Swieten JC, Lee SE. Brain volumetric deficits in MAPT mutation carriers: a multisite study. Ann Clin Transl Neurol. 2021 Jan;8(1):95-110. Epub 2020 Nov 28 PubMed.
- Lee SE, Sias AC, Kosik EL, Flagan TM, Deng J, Chu SA, Brown JA, Vidovszky AA, Ramos EM, Gorno-Tempini ML, Karydas AM, Coppola G, Geschwind DH, Rademakers R, Boeve BF, Boxer AL, Rosen HJ, Miller BL, Seeley WW. Thalamo-cortical network hyperconnectivity in preclinical progranulin mutation carriers. Neuroimage Clin. 2019;22:101751. Epub 2019 Mar 16 PubMed.
- Lee SE, Sias AC, Mandelli ML, Brown JA, Brown AB, Khazenzon AM, Vidovszky AA, Zanto TP, Karydas AM, Pribadi M, Dokuru D, Coppola G, Geschwind DH, Rademakers R, Gorno-Tempini ML, Rosen HJ, Miller BL, Seeley WW. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin. 2017;14:286-297. Epub 2016 Dec 10 PubMed.
- Jiskoot LC, Panman JL, Meeter LH, Dopper EG, Donker Kaat L, Franzen S, van der Ende EL, van Minkelen R, Rombouts SA, Papma JM, van Swieten JC. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain. 2019 Jan 1;142(1):193-208. PubMed.
- Feis RA, van der Grond J, Bouts MJ, Panman JL, Poos JM, Schouten TM, de Vos F, Jiskoot LC, Dopper EG, van Buchem MA, van Swieten JC, Rombouts SA. Classification using fractional anisotropy predicts conversion in genetic frontotemporal dementia, a proof of concept. Brain Commun. 2020;2(2):fcaa079. Epub 2020 Jun 11 PubMed.
- Panman JL, Venkatraghavan V, van der Ende EL, Steketee RM, Jiskoot LC, Poos JM, Dopper EG, Meeter LH, Donker Kaat L, Rombouts SA, Vernooij MW, Kievit AJ, Premi E, Cosseddu M, Bonomi E, Olives J, Rohrer JD, Sánchez-Valle R, Borroni B, Bron EE, Van Swieten JC, Papma JM, Klein S, GENFI consortium investigators. Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2021 May;92(5):494-501. Epub 2021 Jan 15 PubMed.
Further Reading
Papers
- Malpetti M, Jones PS, Tsvetanov KA, Rittman T, van Swieten JC, Borroni B, Sanchez-Valle R, Moreno F, Laforce R, Graff C, Synofzik M, Galimberti D, Masellis M, Tartaglia MC, Finger E, Vandenberghe R, de Mendonça A, Tagliavini F, Santana I, Ducharme S, Butler CR, Gerhard A, Levin J, Danek A, Otto M, Frisoni GB, Ghidoni R, Sorbi S, Heller C, Todd EG, Bocchetta M, Cash DM, Convery RS, Peakman G, Moore KM, Rohrer JD, Kievit RA, Rowe JB, Genfi GF. Apathy in presymptomatic genetic frontotemporal dementia predicts cognitive decline and is driven by structural brain changes. Alzheimers Dement. 2021 Jun;17(6):969-983. Epub 2020 Dec 14 PubMed.
- Olney NT, Ong E, Goh SM, Bajorek L, Dever R, Staffaroni AM, Cobigo Y, Bock M, Chiang K, Ljubenkov P, Kornak J, Heuer HW, Wang P, Rascovsky K, Wolf A, Appleby B, Bove J, Bordelon Y, Brannelly P, Brushaber D, Caso C, Coppola G, Dickerson BC, Dickinson S, Domoto-Reilly K, Faber K, Ferrall J, Fields J, Fishman A, Fong J, Foroud T, Forsberg LK, Gearhart DJ, Ghazanfari B, Ghoshal N, Goldman J, Graff-Radford J, Graff-Radford NR, Grant I, Grossman M, Haley D, Hsiung G, Huey ED, Irwin DJ, Jones DT, Kantarci K, Karydas AM, Kaufer D, Kerwin D, Knopman DS, Kramer JH, Kraft R, Kremers W, Kukull W, Lapid MI, Litvan I, Mackenzie IR, Maldonado M, Manoochehri M, McGinnis SM, McKinley EC, Mendez MF, Miller BL, Onyike C, Pantelyat A, Pearlman R, Petrucelli L, Potter M, Rademakers R, Ramos EM, Rankin KP, Roberson ED, Rogalski E, Sengdy P, Shaw LM, Syrjanen J, Tartaglia MC, Tatton N, Taylor J, Toga A, Trojanowski JQ, Weintraub S, Wong B, Wszolek Z, Boxer AL, Boeve BF, Rosen HJ, ARTFL and LEFFTDS consortia. Clinical and volumetric changes with increasing functional impairment in familial frontotemporal lobar degeneration. Alzheimers Dement. 2020 Jan;16(1):49-59. Epub 2020 Jan 6 PubMed.
- Tsvetanov KA, Gazzina S, Jones PS, van Swieten J, Borroni B, Sanchez-Valle R, Moreno F, Laforce R Jr, Graff C, Synofzik M, Galimberti D, Masellis M, Tartaglia MC, Finger E, Vandenberghe R, de Mendonça A, Tagliavini F, Santana I, Ducharme S, Butler C, Gerhard A, Danek A, Levin J, Otto M, Frisoni G, Ghidoni R, Sorbi S, Rohrer JD, Rowe JB, Genetic FTD Initiative, GENFI. Brain functional network integrity sustains cognitive function despite atrophy in presymptomatic genetic frontotemporal dementia. Alzheimers Dement. 2021 Mar;17(3):500-514. Epub 2020 Nov 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.