Mixed Bag on Tau Tracer Validation, Kinetics: Some Things Fit, Some Don’t
Quick Links
Usually in PET development, before a new tracer is explored in many people and applied in clinical trials, it undergoes extensive validation to determine precisely where it is binding, and kinetic modeling studies to determine how it behaves in the human body over time. At least that is the standard sequence. For tau PET, the urgency of the disease burden—and the competitive advantage of having the first widely used tracer—is perceived to be so great that the leading tracer, T807/AV1451, is already being advanced into Phase 2 studies and readied for use in therapeutic trials before that groundwork is completed. Both fronts are moving in parallel. At the 9th Human Amyloid Imaging conference, held January 14 to 16, scientists reported results of this ongoing work, and debated them extensively.
Several groups presented largely converging results of binding studies in human postmortem brain tissue. Marta Marquie, who works with Teresa Gomez-Isla at Massachusetts General Hospital in Boston, used three different methods to characterize T807/AV1451 in brain slices of people who had died with Alzheimer’s; a tauopathy such as Pick’s disease, progressive supranuclear palsy (PSP), or corticobasal degeneration (CBD); an α-synucleinopathy such as dementia with Lewy bodies (DLB) or multiple system atrophy; or with TDP-43 inclusion pathology. First, Marquie used a fluorescent analogue of T807 and the antibody PHF1 directed against phosphorylated tau. Both bound to neurofibrillary tangles and dystrophic neurites in AD. Both also bound to a variety of expected structures in non-AD tauopathies, such as Pick bodies, astrocytic tau deposits, and dystrophic neurites. T807 did not bind brain tissue from Lewy body diseases or to TDP43 inclusions.
T807 phosphor screen autoradiography painted a similar picture. The characteristic black binding appeared strongly, as a thick band, in the expected cortical regions in AD, but not in controls or slices from the α-synuclein diseases. With this method, however, the non-AD tauopathies were puzzlingly negative.
Finally, Marquie used a beautiful autoradiography method where the T807-exposed slice of brain tissue is dipped into photographic emulsion, left overnight in the dark, and developed much like a Kodak photo. This allows the scientists to visualize T807/AV1451-labeled tissue at subcellular resolution. “With this method we see that T807 perfectly co-localized with tau-containing neurons and with dystrophic neurites,” Marquie told the audience. Like the standard autoradiography, this method, too, showed strong tracer binding to tau pathology in AD but not cerebral amyloid angiopathy, DLB, MSA or TDP-43. The high-resolution images of T807-labeled tangles prompted wide admiration at HAI. In toto, Marquie noted that tangles and dystrophic neurites appear to account for most of the in-vivo T807 signal.
Like Marquie, Val Lowe of the Mayo Clinic in Rochester, Minnesota, reported that T807/AV1451 binding matched up closely with tau PHF-1 but not with TDP-43 immunoreactivity in sections of human brain tissue. “We find exactly what Marta saw,” Lowe said. Lowe showed examples of FTD cases that were due to TDP-43 pathology, none of which bound T807/AV1451. Clinically, those were bvFTD or semantic dementia or PPA, and genetically, they were due to progranulin mutations or unknown causes, but in all cases their postmortem brain showed TDP-43 inclusions, not tau pathology.

[Images courtesy of Val Lowe, Mayo Clinic.]
Top left: Confirmed case of Alzheimer's. Autopsy picture (A) shows macroscopic slab of brain at the level of the amygdala (a). Microscopy sections of the boxed region show strong autoradiography signal with the tau tracer AV1451 (B). Tau antibody staining is strong in amygdala (C, arrow), showing pretangles, tangles, and neuritic threads at high magnification (E). This case had no TDP-43 (D, F.) .
Bottom: Confirmed case of FTLD-TDP. Autopsy picture (A) shows macroscopic slab of brain at the level of the amygdala (arrows in B-D). Microscopy sections of the boxed region show no autoradiography signal with AV1451 (B) or tau antibody staining (C at low, E high magnification). TDP-43 antibody staining was not discernible at low magnification (D) but revealed neuronal cytoplasmic inclusions and neuritic pathology at high magnification (F).
T807/AV1451 not binding TDP-43 inclusions was an important finding at HAI this year, and a relief to the field. Last year, unexpectedly positive T807/AV1451 scans in living patients whose symptoms were thought to be due to TDP-43 had raised the uncomfortable prospect that the new tracer might not distinguish those two major types of protein aggregation. These postmortem studies confirm that it does, said Keith Johnson of Massachusetts General Hospital.
Besides Marquie and Lowe, Yin-Guo Lin of Avid Radiopharmaceuticals of Philadelphia, working with researchers at Northwestern University in Chicago and the Banner Sun Health in Sun City, Arizona, reported similar findings. Lin saw no overlap between a fluorescent form of T807/AV1451 and TDP-43 immunohistochemistry. He did see strong autoradiography in areas rich in tau but not TDP-43 pathology. This postmortem validation study used 50 brain-slice sections of 30 cases with AD, various forms of FTD, and a normal control.
However, there are niggling problems with T807/AV1451, as well. They fall into two separate buckets: unexpected binding to brain regions that make no apparent sense versus absence of expected binding in tau-laden regions, and the behavior of the tracer in the brain during the course of the imaging session. The puzzling binding starts with the apparent absence of signal in some tauopathies such as Pick’s disease or corticobasal degeneration. Both Marquie and Lowe reported this. As explanations, Lowe suggested that T807/AV1451 might bind preferentially to 4-repeat tau over 3-repeat tau, and Marquie thought the tracer might recognize the paired helical filaments of AD more strongly than the straight helical filaments of CBD. At HAI, Dennis Dickson of the Mayo Clinic in Jacksonville, Florida, reviewed the bewildering variety of aggregated forms of tau in the spectrum of known tauopathies (watch his presentation here). PSP is of particular urgency to scientists, as several groups are planning therapy trials in this rare, pure tauopathy; alas, the first few cases being scanned with T807/AV1451 or studied postmortem have not yet generated a clear picture, and more research is needed.
The puzzling binding continues with off-target signals. Several groups using T807/AV1451 reported that it generates a fairly strong signal in parts of the brain’s basal ganglia, e.g., the striatum and substantia nigra, regardless of the patient’s diagnosis. In postmortem slices, Marquie saw this, too, yet she found no tau pathology in adjacent sections. Some groups see a thin line of what they believe is off-target binding at the edge of the hippocampus. T807/AV1451 also appears to bind the choroid plexus in scans. It is unclear where this binding comes from and what it means.
These early observations prompted extensive discussion at HAI. Scientists agreed that a definitive grip on the issue will require more postmortem and ultimately autopsy samples. In the meantime, Gil Rabinovici of the University of California, San Francisco, called on all groups to pool their data and contrast and compare jointly. Applauding this suggestion, Johnson said, “The main story here is that this tracer works well for AD. It shows a strong signal in the expected areas, now confirmed by autoradiography. FTDs are rare and more complex pathologically, so it will take longer to have enough cases to settle these questions.”
The second “bucket” of tracer issues that remain to be worked out concerns the tracer’s kinetics. At HAI, Suzanne Baker of Lawrence Berkeley National Laboratory in Berkeley, California, took a crack at characterizing how T807/AV1451 behaves during the course of a long scanning session. “With PiB a lot of modeling and careful, quantitative in vivo work has been done, but with the tau tracers people are so excited to get them into trials that this technical work lags behind. It is important to do that if you want to measure small changes over time or in response to a drug, especially in a multicenter trial,” she told Alzforum.
Baker scanned 19 older healthy controls and 14 people with AD for 150 minutes (with a 20-minute break before the last half-hour) and analyzed how the tracer distributed between different brain areas and how that changed over time. Her key finding was that in AD patients, different brain regions had different kinetics. In particular, T807/AV1451 in the cortex did not reach a steady state, i.e., a window of time during which the ratio of binding in a target region (i.e. temporal cortex) to binding in the reference tissue (i.e. cerebellum) was stable. This is the most robust time frame for imaging because scientists will get a reproducible measurement from scan to scan. Instead, the kinetics of T807/AV1451 turned out to be fast in subcortical regions where the tracer is thought to have some off-target binding, intermediate in other regions, and so slow in the cortical regions—perhaps because they contain the most tau—that their uptake never stabilized in the 150-minute scanning period. “We were astonished by the variability across regions that we saw,” Baker said.
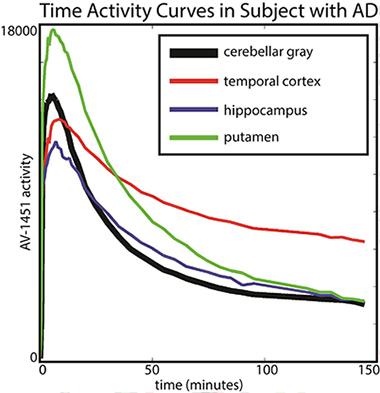
The putamen shows faster kinetics and the temporal cortex shows slower kinetics in comparison to cerebellar gray (reference region). [Image courtesy of Suzanne Baker and William Jagust.]
This finding essentially implies that the tracer’s uptake is a moving target. For example, a scan taken between 80 and 100 minutes after injection might give a different result than one taken between 120 and 140 minutes after injection, raising questions about how best to compare tracer uptake between different brain regions and in longitudinal studies.
These uncertainties do not question the tracer’s ability to detect whether there is a significant amount of tau pathology in a given brain region, only the finer quantitative measurements, Baker said. The problems require careful attention to issues of timing of scan acquisition.
Baker’s talk prompted extensive discussion and some jokes about 12-step programs. “The first step is admitting you have a problem,” she quipped. Others agreed that more kinetic modeling can help solve it, ideally with a study that uses arterial input data to better approximate how much tracer enters the brain at which time point after injection. Some kinetic modeling studies are underway at Avid Radiopharmaceuticals and at MGH.
In summary, Chet Mathis of the University of Pittsburgh recommended that all tau tracers that will be used clinically be subjected to both careful ex vivo validation and pharmacokinetic modeling to clarify their specific binding and distribution between regions and compartments in the brain. “We have to do the hard work before we can interpret the scans,” Mathis said.
On the other hand, PET experts also largely subscribe to the notion embodied by a quote by former Defense Secretary Donald Rumsfeld: “You go to war with the army you have … not the army you might want or wish to have at a later time.” For FDA approval, PET tracers will most likely need autopsy correlations, which have started but take a long time to obtain. Scientists are beginning to suspect that tau’s close correlation to neurodegeneration and clinical symptoms means tau PET might prove to be a good outcome marker in therapy trials, whereas amyloid PET might be more useful as an inclusion criteria at screening, and they are eager to test whether this is true. “Tau PET measures a molecular pathology, and it is close to the action. That combination has been missing,” Johnson told Alzforum.
Bill Klunk of UPitt, who co-developed PiB with Mathis, emphasized that none of the technical questions with T807/AV1451 are show-stoppers. “Let’s not throw the baby out with the bathwater. Let’s just clean up the bathwater. When you look at the overall results with T807 so far, it’s clear the baby is still really good,” Klunk said.—Gabrielle Strobel
References
Antibody Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.