UB-312 Synuclein Vaccine Safe in Controls. Next Up: Parkinson's.
Quick Links
Current treatments for Parkinson’s only address the disease's symptoms, and much like Alzheimer's scientists are pursuing that disease's central pathogenic proteins, Parkinson's scientists believe that targeting α-synuclein aggregates will slow or stop the movement disorder from getting worse. Following in the footsteps of α-synuclein antibodies, such as Roche/Prothena’s prasinezumab, scientists are testing active vaccines in the clinic. At the 16th International Conference on Alzheimer’s and Parkinson’s Diseases held online and in Barcelona, Spain, Hui Jing Yu of Vaxxinity, Dallas, reported that in a Phase 1 trial in healthy volunteers, UB-312 spurred production of C-terminal synuclein antibodies without causing serious side effects. The company is now enrolling people with Parkinson’s for a second trial. UB-312 is the second α-synuclein vaccine in the clinic; ACI-7104, developed by AFFiRiS and then licensed to AC Immune, also appeared safe in Phase 1.
- UB-312 caused no serious adverse events in healthy volunteers.
- In mice, a vaccine sporting two α-synuclein fragments induced more antibodies than those with either peptide alone.
- A bispecific antibody against α-synuclein and the transferrin receptor crossed the mouse BBB, altered pathology.
- Toll-like receptor 2 immunotherapy reduced synuclein load, restored motor function in mice.
Nipping at their heels are a pack of other α-synuclein immunotherapy approaches. In Barcelona, Robin Barbour of Prothena, San Francisco, showed how a vaccine carrying two C-terminal α-synuclein fragments jolted mice into making more antibodies than either snippet alone did. Those antibodies labeled Lewy bodies in brain tissue from PD cases. Sahar Roshanbin from Uppsala University, Sweden, showed how a hybrid α-synuclein/transferrin receptor antibody slipped into the mouse brain better than did the synuclein antibody alone. Chasing a different target, Jun Sung Lee of Neuramedy, a small biotech in Seoul, South Korea, reported that tomaralimab, an antibody against toll-like receptor 2, lowered α-synuclein aggregate load, neuroinflammation, and motor dysfunction in a mouse model of synucleinopathy.
The need for taking many shots on goal is pressing. “When you don’t have any disease-slowing therapies to keep people out of the late stages of Parkinson’s where dementia may develop, you want to try everything,” Mark Cookson, National Institute on Aging, Baltimore, told Alzforum.
Active Synuclein Immunotherapy
For one, the α-synuclein vaccine field is stirring. From 2012 to 2017, AFFiRiS’ PD01A/PD03A, based on an eight-amino acid fragment from the C-terminus, showed some safety and capacity to provoke an antibody response in Phase 1 trials in PD and multiple system atrophy (MSA, Volc et al., 2020; Meissner et al., 2020). The program stalled until July 2021, when AC Immune acquired rights and said it would start Phase 2 in a formulation called ACI-7104. According to the company website, this trial is to measure immunogenicity, imaging and fluid biomarkers, and progression of motor and non-motor symptoms in people with early sporadic PD, but no trials are listed on clinicaltrials.gov.
Meanwhile, UB-312 is catching up. Vaxxinity's vaccine links a 10-amino-acid fragment from α-synuclein's C-terminus to a small peptide that activates T-helper cells. In guinea pigs, a 12-amino-acid predecessor of UB-312 had generated antibodies against toxic α-synuclein fibrils and oligomers, but not monomers. In postmortem analysis, these antibodies bound synuclein inclusions within the substantia nigra and basal ganglia of people who had had PD, dementia with Lewy bodies, or MSA (Nimmo et al., 2020).
As for UB-312, when injected into 10-week-old Line 61 transgenic mice that express human α-synuclein, fewer α-synuclein oligomers formed in the cortex, hippocampus, and striatum. Vaccinated mice did better at crossing a beam and hanging onto a wire (Nimmo et al., 2022).
At AD/PD, Yu shared safety and immunogenicity data from 50 healthy adults in a Phase 1 trial conducted in the Netherlands. Participants had gotten intramuscular jabs at weeks 1, 5, and 13, following one of seven dosing schedules: either three doses each of 40, 100, 300, 1,000, or 2,000 μg, or 40 μg initially followed by either 300 or 1,000 μg for the last two doses. Six people received each vaccine regimen, eight a placebo. Volunteers donated CSF at baseline and week 21, and blood at baseline, one week after each jab, and every four weeks from week 1 to 21. All were monitored for adverse events for another 23 weeks.
Doses at or below 300 μg prompted no serious adverse events, but did cause mild headache, cold-like complaints, and pain or redness at the injection site. The dose-ranging stopped at the 1,000 and 2,000 μg doses, after one volunteer developed moderate flu-like symptoms after the second 1,000 μg injection.
UB-312 prompted a dose-dependent rise in α-synuclein antibodies in the blood and CSF, with the largest response coming after three 300 μg doses (see image below). The antibody concentration in the CSF reached only 0.2 percent of that in the blood, indicating that a small fraction had crossed the blood-brain barrier. Yu considers this good news, as it is on par with the reported serum-to-CSF ratios of 0.1 to 0.2 percent for monoclonal antibodies. [Editor's note: This data was published on April 15, 2022, in the journal Movement Disorders.]
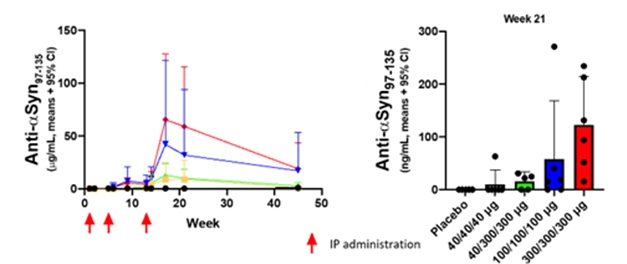
Immune Response. Healthy adults who received three injections of 300 μg (red) or 100 μg (blue) UB-312 made more C-terminal α-synuclein antibodies in the blood (left) and CSF (right) than volunteers who got lower doses (yellow and green) or placebo (black). [Courtesy of Vaxxinity.]
Still in Phase 1, the researchers are now testing an additional 20 people ages 40 to 85 with early to mid-stage sporadic Parkinson's. Yu expects enrollment to wrap up by the end of April. These participants, too, will get three doses of either 100 or 300 μg UB-312 over the same 13-week period. Seven will receive each dose of active vaccine; six, placebo. Endpoints include change in the Unified Parkinson Disease Rating Scale and the Montreal Cognitive Assessment, and blood and CSF will be collected and monitored for anti-α-synuclein antibodies, as well as changes in total and free α-synuclein.
Unlike for AD, there are no established CSF or serum biomarkers for PD. Instead, the researchers will measure misfolded pathogenic synuclein before and after vaccination using the Protein Modification Cyclic Amplification technique. PMCA can detect minuscule amounts of α-synuclein oligomers in CSF from people with PD and other synucleinopathies (Dec 2016 news; Kang et al., 2019). “This method might help to diagnose these neurological disorders and provide a way to assess target engagement of PD treatments,” Yu said, adding that topline results could come by the end of this year.
Stronger in Tandem?
Both PD01A/PD03A and UB-312 contain two antigens—one against synuclein and one to stimulate T-cells. Can packing on more boost the immune response? Prothena has bumped the antigen count to three. At AD/PD, Barbour said the strategy is to deploy vaccines with a T-cell-stimulating epitope and two α-synuclein fragments separated by a dendritic endopeptidase cleavage site. Prothena is doing this for its dual Aβ-tau vaccine, as well (Aug 2021 conference news). Michael Agadjanyan, Institute for Molecular Medicine, Huntington Beach, California, told Alzforum he is unaware of other vaccines that include such a cleavage site between antigenic fragments and wondered why this is needed. “Dendritic cells chop up antigens into small fragments to present to T-cells regardless of an endopeptidase cleavage site,” he told Alzforum
Still, the trick might work. Barbour has tested six different vaccines using two C-terminal and one mid-domain fragment. One vaccine has both C-terminal fragments, two have the mid-domain tethered to either of the C-terminal antigens, and three comprise single peptides. In wild-type mice, the tandem vaccines evoked seven to 10 times higher antibody titers than the single-antigen varieties, even when the latter were given at twice the dose. Tandem vaccine antisera more robustly labeled Lewy bodies in Parkinson's brain tissue, and prevented cultured neurons from taking up recombinant, fluorescent α-synuclein aggregates. Tandem C-terminal vaccine antisera bound Lewy bodies and prevented synuclein uptake by cells better than did antisera from the C- and mid-domain fragment vaccines. Barbour said the dual C-terminal vaccine has been selected for clinical testing, though she did not say when.
Why stop at three antigens? Agadjanyan and colleagues are pushing the envelope by creating vaccines with 13 to 15 epitopes, including 12 antigenic peptides from infectious pathogens that stimulate T-cells. “This could induce a robust immune response in almost everyone, since each person has their own set of MHC class II molecules on their antigen-presenting cells that could recognize some, but not all, of the antigens,” he said. The scientists have developed a DNA-based, multi-fragment, α-synuclein vaccine (Kim et al., 2022; Davtyan et al., 2017). It contains a plasmid carrying genes for the 12 T-cell epitopes and three different α-synuclein fragments spanning the entire C-terminus. Once translated, the peptides run as one long string with no cleavage sites in between.
Much like the Prothena tandem vaccine, this DNA-based jab induced higher antibody titers in mice than vaccines encoding each α-synuclein fragment alone. Antibodies created by the multi-fragment version also bound Lewy bodies in postmortem brain tissue. The vaccine reduced the amount of total α-synuclein deposits in the brains of transgenic mice, slowed neuron loss, reduced micro- and astrogliosis, and improved motor function better than any single-fragment vaccines. Agadjanyan hopes to develop a protein-based multi-fragment vaccine as well.
Targeting Synuclein Using Antibodies
In parallel, researchers are testing therapeutic antibodies, as well. Such biologic drugs against α-synuclein have been in clinical development for more than a decade and seem safe, though it's still unclear if they work. Roche/Prothena’s prasinezumab is the furthest along in Phase 2 trials (see Mar 2013 conference news; Mar 2015 conference news). Just as vaccines against synuclein’s C-terminus seem promising, antibodies that recognize this portion of the peptide, such as prasinezumab and Lundbeck’s LuAF82422, have fared well in early trials. In contrast, the only antibody tested so far against the N-terminus, Biogen’s cinpanemab, posted negative results in Phase 2 (Apr 2021 conference news).
Whichever candidate antibody will succeed, more of it needs to get into the brain. In Barcelona, Roshanbin of Uppsala University described a tweaked version of the SynO2, which binds oligomeric and fibrillar α-synuclein species that are believed to be most toxic (Vaikath et al., 2015; Ingelsson, 2016).
Initially, Roshanbin aimed to develop an antibody-based PET ligand for α-synuclein. To drive SynO2 uptake into the brain, she tacked on an antibody fragment that binds the transferrin receptor. TfR transports transferrin across cells that line the blood-brain barrier, and scientists have piggybacked other cargoes onto the receptor to get them in (Dec 2021 conference news; Mar 2021 conference news; May 2020 news). Lo and behold, a radiolabeled version of the SynO2-TfR bispecific antibody reached levels in the brain 50 times higher than did SynO2, and, in PET scans, it lit up extracellular α-synuclein deposits in transgenic mice (see image below; Roshanbin et al., 2022).

Antibody PET. MicroPET scans of mouse brains show that more SynO2-TfR (left) than SynO2 (right) was able to cross the blood-brain barrier. [Courtesy of Sahar Roshanbin, Uppsala University.]
Roshanbin wondered if more uptake would mean a better therapeutic effect. She injected Line 61 mice with SynO2-TfR or SynO2 on days 1, 3, and 4, then sampled the brain on day 5. Compared to SynO2, the bispecific antibody lowered total α-synuclein and insoluble oligomer levels but increased the amount of soluble oligomers, hinting that the antibody was destabilizing or preventing synuclein aggregates.
Since microglia mop up antibody-antigen complexes in the brain, the scientists looked for signs of microglial activity. In mice injected with either antibody, brain levels of soluble TREM2 rose, as did the number of Iba1-positive cells, both signs of microglial activation. This was only a five-day experiment, hence the phosphorylated α-synuclein deposits did not budge. Roshanbin is planning longer experiments to track changes in pathology and see if the microglia better phagocytose α-synuclein when mobilized.
Last but not least, Neuramedy’s Lee described a different way to attack α-synuclein deposits. This company is developing tomaralimab, an antibody to toll-like receptor 2. In the brain, TLR2 ushers α-synuclein aggregates inside neurons, while it triggers neuroinflammation in microglia (Apr 2020 conference news; Oct 2020 news; Kim et al., 2021). A small molecule inhibitor of TLR2 signaling, NPT520-34, reduced α-synuclein aggregates and improved motor symptoms in Line 61 mice. In a Phase 1 trial for PD, it caused no serious adverse events in healthy adults.
Tomaralimab, aka OPN-305, was developed by Opsona Therapeutics, Ireland. It is the only TLR2 antibody that was temporarily in clinical development, between 2012 and 2019, with three Phase 1 studies for cancer and kidney transplant recipients (see clinicaltrials.gov). Neuramedy bought the rights to this antibody in 2019.
At AD/PD, Lee reported that tomaralimab reduced expression of the inflammatory cytokines interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) in cultured microglia, and reduced the propagation of α-synuclein between cells.
To see if these effects held true in vivo, the researchers turned to a mouse model of synucleinopathy (Nov 2012 news). They injected preformed synuclein fibrils into 10-week-old wild-type mice to seed pathology and trigger neurodegeneration. Beginning a week later, they infused tomaralimab or placebo once weekly for 18 weeks, then assessed synuclein pathology and neuroinflammation. The antibody entered the brain, reaching CSF levels 0.1 percent of that in plasma. Phosphorylated α-synuclein aggregates, Iba1-positive microglia, and GFAP-positive astrocytes were less in treated mice than controls, Lee reported at AD/PD. Antibody treatment soothed inflammation, as well, with mice having fewer IL-1β-, IL-6-, and TNF-α-positive cells. Line 61 transgenic mice given the same tomaralimab regimen showed similar results.
Furthermore, the antibody improved muscle strength, motor activity, and coordination of the fibril-injected mice. Treated animals held onto a wire for longer, scurried farther in an open field, and spent more time walking a balance beam than controls. Lee said these results seem promising, but not whether Neuramedy will evaluate tomaralimab in PD.
Both Cookson and Martin Ingelsson, who is now at the University of Toronto, consider anti-synuclein vaccines and antibodies equally valid approaches. Ingelsson, who works with Roshanbin at Uppsala U, pointed out a wrinkle unique to vaccines against α-synuclein. “While AD seems to only engage the brain, PD affects the whole nervous system and includes peripheral protein deposits, so researchers need to ensure that α-synuclein species in vaccines will not potentiate peripheral and, secondarily, central neuropathy” he told Alzforum.
Cookson thinks the biggest challenge may be that the body tolerates only mild swings in α-synuclein level. “Three copies of the normal SNCA allele cause an early onset, aggressive form of Parkinson’s with a lot of cortical involvement, while lowering synuclein brain levels too much also causes cognitive impairment,” Cookson told Alzforum (Nov 2003 news). While vaccines are easier to administer than monthly antibody infusions, Cookson likes antibodies because they target only toxic α-synuclein species while leaving the normal protein alone, which lessens the concern about driving synuclein levels down too much.—Chelsea Weidman Burke
References
Therapeutics Citations
Research Models Citations
News Citations
- Methods to Detect Amyloid Seeds Improve, Extend to Blood and Parkinson’s
- Up-and-Coming Immunotherapies Target Aβ and Tau
- Like Star Born of Supernova, Plaque Born of Exploded Neuron?
- Antibody Against α-Synuclein Looks Safe In Phase 1
- For α-Synuclein Immunotherapy, Is Going Later the Key?
- Brain Shuttle Could Halve Amount of Gantenerumab Needed
- Shuttle Unloads More Gantenerumab Into the Brain
- Molecular Transport Vehicle Shuttles Therapies into Brain
- Parkinson's Therapies Seek to Stem Progression
- α-Synuclein Spurs Neuroinflammation Via Microglial LRRK2
- Toxic Synuclein Corrupts Native in Wild-Type Mice
- Synuclein and Parkinson's—It's All in the Dose
Paper Citations
- Volc D, Poewe W, Kutzelnigg A, Lührs P, Thun-Hohenstein C, Schneeberger A, Galabova G, Majbour N, Vaikath N, El-Agnaf O, Winter D, Mihailovska E, Mairhofer A, Schwenke C, Staffler G, Medori R. Safety and immunogenicity of the α-synuclein active immunotherapeutic PD01A in patients with Parkinson's disease: a randomised, single-blinded, phase 1 trial. Lancet Neurol. 2020 Jul;19(7):591-600. PubMed.
- Meissner WG, Traon AP, Foubert-Samier A, Galabova G, Galitzky M, Kutzelnigg A, Laurens B, Lührs P, Medori R, Péran P, Sabatini U, Vergnet S, Volc D, Poewe W, Schneeberger A, Staffler G, Rascol O, AFF009 Study Investigators. A Phase 1 Randomized Trial of Specific Active α-Synuclein Immunotherapies PD01A and PD03A in Multiple System Atrophy. Mov Disord. 2020 Nov;35(11):1957-1965. Epub 2020 Sep 3 PubMed.
- Nimmo JT, Verma A, Dodart JC, Wang CY, Savistchenko J, Melki R, Carare RO, Nicoll JA. Novel antibodies detect additional α-synuclein pathology in synucleinopathies: potential development for immunotherapy. Alzheimers Res Ther. 2020 Nov 30;12(1):159. PubMed. Correction.
- Nimmo JT, Smith H, Wang CY, Teeling JL, Nicoll JA, Verma A, Dodart JC, Liu Z, Lin F, Carare RO. Immunisation with UB-312 in the Thy1SNCA mouse prevents motor performance deficits and oligomeric α-synuclein accumulation in the brain and gut. Acta Neuropathol. 2022 Jan;143(1):55-73. Epub 2021 Nov 6 PubMed.
- Kang UJ, Boehme AK, Fairfoul G, Shahnawaz M, Ma TC, Hutten SJ, Green A, Soto C. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson's disease. Mov Disord. 2019 Apr;34(4):536-544. Epub 2019 Mar 6 PubMed.
- Kim C, Hovakimyan A, Zagorski K, Antonyan T, Petrushina I, Davtyan H, Chailyan G, Hasselmann J, Iba M, Adame A, Rockenstein E, Szabo M, Blurton-Jones M, Cribbs DH, Ghochikyan A, Masliah E, Agadjanyan MG. Efficacy and immunogenicity of MultiTEP-based DNA vaccines targeting human α-synuclein: prelude for IND enabling studies. NPJ Vaccines. 2022 Jan 10;7(1):1. PubMed. Correction.
- Davtyan H, Zagorski K, Petrushina I, Kazarian K, Goldberg NR, Petrosyan J, Blurton-Jones M, Masliah E, Cribbs DH, Agadjanyan MG, Ghochikyan A. MultiTEP platform-based DNA vaccines for alpha-synucleinopathies: preclinical evaluation of immunogenicity and therapeutic potency. Neurobiol Aging. 2017 Nov;59:156-170. Epub 2017 Aug 10 PubMed.
- Vaikath NN, Majbour NK, Paleologou KE, Ardah MT, van Dam E, van de Berg WD, Forrest SL, Parkkinen L, Gai WP, Hattori N, Takanashi M, Lee SJ, Mann DM, Imai Y, Halliday GM, Li JY, El-Agnaf OM. Generation and characterization of novel conformation-specific monoclonal antibodies for α-synuclein pathology. Neurobiol Dis. 2015 Jul;79:81-99. Epub 2015 Apr 30 PubMed.
- Ingelsson M. Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson's Disease and Other Lewy Body Disorders. Front Neurosci. 2016;10:408. Epub 2016 Sep 5 PubMed.
- Roshanbin S, Xiong M, Hultqvist G, Söderberg L, Zachrisson O, Meier S, Ekmark-Lewén S, Bergström J, Ingelsson M, Sehlin D, Syvänen S. In vivo imaging of alpha-synuclein with antibody-based PET. Neuropharmacology. 2022 May 1;208:108985. Epub 2022 Feb 8 PubMed.
- Kim C, Kwon S, Iba M, Spencer B, Rockenstein E, Mante M, Adame A, Shin SJ, Fields JA, Rissman RA, Lee SJ, Masliah E. Effects of innate immune receptor stimulation on extracellular α-synuclein uptake and degradation by brain resident cells. Exp Mol Med. 2021 Feb;53(2):281-290. Epub 2021 Feb 16 PubMed.
Other Citations
External Citations
Further Reading
Papers
- Kim C, Spencer B, Rockenstein E, Yamakado H, Mante M, Adame A, Fields JA, Masliah D, Iba M, Lee HJ, Rissman RA, Lee SJ, Masliah E. Immunotherapy targeting toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating α-synuclein transmission and neuroinflammation. Mol Neurodegener. 2018 Aug 9;13(1):43. PubMed.
Primary Papers
- Yu HJ, Thijssen E, van Brummelen E, van der Plas JL, Radanovic I, Moerland M, Hsieh E, Groeneveld GJ, Dodart JC. A Randomized First-in-Human Study With UB-312, a UBITh® α-Synuclein Peptide Vaccine. Mov Disord. 2022 Jul;37(7):1416-1424. Epub 2022 Apr 15 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.