Abnormal Tau Slips into Synapses Long Before Tangles Form
Quick Links
In mice, data suggest that tau seeds sneak across synapses, spreading tangle pathology throughout the brain. Is the same true in people? Quite likely, say scientists led by Tara Spires-Jones, University of Edinburgh, U.K., and Alberto Lleó, Hospital of Sant Pau, Barcelona, Spain. Using high-resolution array tomography, they zoomed in on synapses in postmortem brain tissue from people who had had Alzheimer’s disease. In the May 15 Neuron, they reported that phosphorylated, misfolded, and oligomeric tau had gathered at presynapses, even in areas with few neurofibrillary tangles, suggesting that this form of tau accumulates early in tauopathy. Tau was also spotted in postsynapse. The data indirectly support the idea that toxic tau species spread across the synaptic cleft, and through the brain along connectivity pathways, though the researchers cannot capture that movement in postmortem tissue.
“This study presents compelling structural evidence that accumulation of tau oligomers at presynaptic terminals is an early event in Alzheimer’s disease neuropathology, which may lead to anterograde transsynaptic spreading, then to the regional distribution of tau neuropathology later in disease,” wrote Agenor Limon of the University of Texas Medical Branch, Galveston (comment below). Dezhi Liao of the University of Minnesota, Minneapolis, noted that tau is enriched in axons and presynaptic terminals in healthy cells, but moves to postsynaptic structures in neurodegenerative diseases, based on cellular and animal studies. “This is the first evidence directly proving that tau is increased in postsynaptic terminals in human AD tissue,” Liao told Alzforum.
As described by neuropathological Braak staging and in vivo tau PET scans, neurofibrillary tangles progress through the brain in a predictable pattern from the brain stem and trans-entorhinal cortex to the limbic system then neocortex through neural circuits (Braak and Braak, 1991; Jan 2020 news). In mouse models of tauopathy, as well, tangles spread among functionally connected neurons, suggesting trans-synaptic travel (Feb 2012 news; de Calignon et al., 2012).
To see if this was true in people, co-first authors Martí Colom-Cadena and Caitlin Davies, both in Edinburgh, analyzed cortical tissue from 24 people who had had AD and 19 healthy adults from the Alzheimer Scotland Brain and Tissue Bank, and the University of Edinburgh Sudden Death Brain Bank, respectively. Most were in their 70s or 80s when they died.
The scientists probed ultrathin 70 nm serial slices of cortex with three fluorescent antibodies: AT8 for phosphorylated tau, Alz50 for misfolded tau, and T22, which preferentially small aggregates over monomers or fibrils of tau (Nov 2010 conference news; Lasagna-Reeves et al., 2012).
Then, using array tomography, the researchers reconstructed 1.3 million pre- and postsynaptic pairs of excitatory neurons. This high-resolution technique stacks two-dimensional images to create three-dimensional representations (see image below). “The ability to see inside individual pre- and postsynaptic terminals is what is unique about this study,” Spires-Jones told Alzforum.
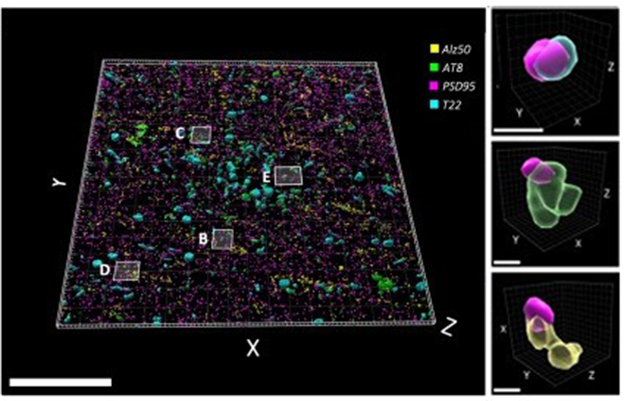
Tau in the Postsynapse. In a sectional array of postmortem brain tissue from a person who died with Alzheimer’s, postsynapses (purple) contained tau oligomers (blue), phospho-tau (green), or misfolded tau (yellow). [Courtesy of Colom-Cadena et al., Neuron, 2023.]
While all three types of tau were spotted on both sides of synapses in AD cases, T22-reactive tau was the most abundant. It was more common in pre- than postsynapses and appeared within presynaptic vesicles. While T22 detected tau bound cortical tissue packed with neurofibrillary tangles, it also picked up tau-packed presynapses in areas with few or no tangles (see image below). This hints that oligomers accumulate there early in tauopathies. Indeed, AD synapses had 3.3 times more T22-reactive tau than p-tau231, an early marker of tau pathology.

With or Without Tangles. In areas with both few (top) and many (bottom) neurofibrillary tangles (left), oligomeric tau (yellow in this figure) accumulates in the presynapses (purple in this figure). [Courtesy of Colom-Cadena et al., Neuron, 2023.]
To the authors' minds, the abundance of T22 tau in pre- versus postsynapses implies neuron-to-neuron transmission, where tau accumulates in the presynapses before being released and captured by the postsynapses (see image below). While this cannot be proven by examining postmortem human tissue, the scientists saw such a pattern in 18-month-old tauopathy mice expressing human P301L in neurons of their entorhinal cortices (Pickett et al., 2017). T22 bound both sides of given synapses. “Since we saw oligomers in the postsynapses, we knew they had to have come from the presynapses,” Spires-Jones said.
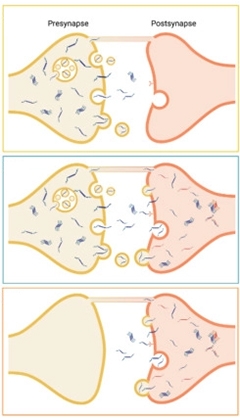
Presynaptic Spread. In this model, tau oligomers accumulate in a presynapse before being released, traveling to the postsynapses. The model depicts three different mechanisms of spread. [Courtesy of Colom-Cadena et al., Neuron, 2023.]
The idea that tau spreads trans-synaptically is not universally accepted. Some researchers think toxic tau could arise by other means, such as de novo in postsynaptic cells under stress (April 2016 webinar; Walsh and Selkoe, 2016).
Others questioned whether T22 specifically recognizes oligomers in this context, since the antibody shows some affinity for tau filaments. “It is possible T22 recognized tau filaments in the synapses studied by Colom-Cadena et al.,” wrote Ryskeldi-Falcon. “Additional studies are required to identify the molecular species of tau present in synapses in the brains of individuals with AD.” T22 recognizes aggregates of tau seeded in vitro by Aβ, but the exact conformation of those forms remains unknown.
Immunoelectron microscopy and synaptoneurosome analysis carried out by Spires-Jones’ group suggest at least some of the tau in synapses is oligomeric. The former identified globular, not fibrillar, deposits in pre- and postsynapses in AD tissue, while western blots of synaptoneurosomes indicated the oligomer-to-monomer ratio in AD synapses was 10-fold higher than in control synapses.
Spires-Jones is now exploring how oligomeric tau spreads between synapses and how it can be stopped.—Chelsea Weidman Burke
References
News Citations
- Connectivity, Not Proximity, Predicts Tau Spread
- Mice Tell Tale of Tau Transmission, Alzheimer’s Progression
- San Diego: Tau Oligomer Antibodies Relieve Motor Deficits in Mice
Antibody Citations
Research Models Citations
Webinar Citations
Paper Citations
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59. PubMed.
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012 Feb 23;73(4):685-97. PubMed.
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Sarmiento J, Troncoso J, Jackson GR, Kayed R. Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J. 2012 Jan 17; PubMed.
- Pickett EK, Henstridge CM, Allison E, Pitstick R, Pooler A, Wegmann S, Carlson G, Hyman BT, Spires-Jones TL. Spread of tau down neural circuits precedes synapse and neuronal loss in the rTgTauEC mouse model of early Alzheimer's disease. Synapse. 2017 Jun;71(6) Epub 2017 Mar 6 PubMed.
- Walsh DM, Selkoe DJ. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci. 2016 Apr;17(4):251-60. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Colom-Cadena M, Davies C, Sirisi S, Lee JE, Simzer EM, Tzioras M, Querol-Vilaseca M, Sánchez-Aced É, Chang YY, Holt K, McGeachan RI, Rose J, Tulloch J, Wilkins L, Smith C, Andrian T, Belbin O, Pujals S, Horrocks MH, Lleó A, Spires-Jones TL. Synaptic oligomeric tau in Alzheimer's disease - A potential culprit in the spread of tau pathology through the brain. Neuron. 2023 Jul 19;111(14):2170-2183.e6. Epub 2023 May 15 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Texas Medical Branch at Galveston
Colom-Cadena’s study presents compelling structural evidence suggesting that accumulation of tau oligomers at presynaptic terminals is an early event in Alzheimer’s disease neuropathology, which may lead to anterograde transsynaptic spreading first, and to the regional distribution of tau neuropathology later in disease.
In addition to the technological elegance of the experiments and the finding that oligomeric forms of tau are a major component in synapses compared with other phosphorylated or misfolded tau forms, this work also suggests a potential mechanism to explain how tau spreading is an activity-dependent phenomenon, which has important relevance for therapeutical approaches in development, including noninvasive ones. Because the technical advance in this study can be used to target specific synapses, it will be very interesting to see future studies where additional types of synapses, e.g., inhibitory, are studied using the same approach and technology.
VIB-KU Leuven Center for Brain and Disease
In this outstanding paper, Colom-Cadena and colleagues present a beautiful set of array tomography and immunoelectron microscopy experiments showing accumulation of oligomeric tau in the synapses of AD patients. Over the last years, several studies have highlighted the critical importance of synaptic tau for the development and progression of pathology in animal models (Hoover et al., 2010; Largo-Barrientos et al., 2021; Tai et al., 2012). Previous work from the groups of Karen Duff and Tara Spires-Jones already showed that pathogenic tau can spread from pre- to postsynapses in the mouse brain (Liu et al., 2012; Pickett et al., 2017). Now, this article provides the first-time evidence of trans-synaptic spread of oligomeric tau in the human brain.
The authors use three antibodies to label oligomeric (T22), misfolded (Alz50), and phosphorylated (AT8) tau and find all three species in synapses of AD brains, with a higher presence of oligomeric tau. T22-positive tau oligomers are found on presynaptic vesicles as well as near the postsynaptic density. Interestingly, oligomeric tau is sometimes associated with fibrils in neurofibrillary tangles (NFTs), but synaptic tau oligomers are preferentially found in areas with no (or low numbers of) NFTs. Finally, synaptic tau oligomers are more frequently found only at the presynapse, followed by at both the pre- and postsynapse, while being only at the postsynapse was rare. This suggests to the authors that propagation of tau oligomers occurs from pre- to postsynapse.
Whether soluble or aggregated tau is the major source of toxicity has been debated for years. Tau oligomers are somehow controversial, and represent a heterogenous intermediate state of soluble tau species with seeding capacity. This study suggests that oligomeric tau species are at least relevant for the progression of the pathology at early stages. Further biochemical and structural characterization of the oligomers that the authors find enriched in synapses of AD brains will be essential to strengthen their findings.
Another open question in the field is how does tau pathology propagate throughout the different brain regions: Do seeds physically move from one region to another (and if so, how?) or do different brain regions become subsequently vulnerable and start accumulating pathogenic tau species? This work provides indirect evidence supporting the first option, with oligomeric tau being transmitted from one neuron to the next in a trans-synaptic (from pre- to postsynapse) pattern. However, the precise mechanisms by which this transmission occurs in the AD brain (released with synaptic vesicles, through lysosomal exocytosis, in exosomes, or transported in extracellular vesicles or via nanotubes, etc.) remain to be fully elucidated.
References:
Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010 Dec 22;68(6):1067-81. PubMed.
Largo-Barrientos P, Apóstolo N, Creemers E, Callaerts-Vegh Z, Swerts J, Davies C, McInnes J, Wierda K, De Strooper B, Spires-Jones T, de Wit J, Uytterhoeven V, Verstreken P. Lowering Synaptogyrin-3 expression rescues Tau-induced memory defects and synaptic loss in the presence of microglial activation. Neuron. 2021 Mar 3;109(5):767-777.e5. Epub 2021 Jan 19 PubMed.
Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012 Oct;181(4):1426-35. PubMed.
Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7(2):e31302. PubMed.
Pickett EK, Henstridge CM, Allison E, Pitstick R, Pooler A, Wegmann S, Carlson G, Hyman BT, Spires-Jones TL. Spread of tau down neural circuits precedes synapse and neuronal loss in the rTgTauEC mouse model of early Alzheimer's disease. Synapse. 2017 Jun;71(6) Epub 2017 Mar 6 PubMed.
MRC Laboratory of Molecular Biology
This study represents an impressive use of fluorescence light microscopy to image and quantify tau within thousands of synapses in postmortem brain tissue from a large number of individuals with AD. The dysfunction and loss of synapses are early pathological changes in AD and are highly correlated with cognitive decline (Terry et al., 1991). It is hypothesized that assembled tau within synapses may mediate their dysfunction and loss. In addition, assembled tau may traverse synapses to spread within connected brain regions.
The authors found tau within pre- and postsynaptic compartments in individuals with AD, which was greatly elevated compared to control individuals. The authors detected abnormally phosphorylated synaptic tau using the antibodies AT8 (pS202 and pT205 tau) and AT180 (pT231 tau), as well as tau immunoreactive against the antibody Alz50. The latter antibody was raised against brain homogenates from individuals with AD and recognizes a discontinuous epitope present on tau filaments (Jicha et al., 1997).
The present results extend previous studies of mouse models (Kopeikina et al., 2013) and of synapses fractionated from postmortem brain tissue (Fein et al., 2008). The quantitative approach of Colom-Cadena et al. revealed an asymmetric distribution of synaptic tau favoring the presynaptic compartment. In addition, it was rare for tau to be present in the postsynaptic compartment in the absence of presynaptic tau. These results suggest that tau may preferentially accumulate in the presynaptic compartment and may subsequently induce tau accumulation in the postsynaptic compartment, possibly by direct transfer.
The authors conclude that oligomeric tau is present in synapses based on labelling with the antibody T22. This antibody, which was raised against recombinant tau seeded with synthetic Aβ42, was reported to detect an oligomeric, non-filamentous form of tau in the soluble brain fraction of individuals with AD (Lasagna-Reeves et al., 2012). However, T22 also exhibited some reactivity against tau filaments in that study (Lasagna-Reeves et al., 2012). In addition, the soluble brain fraction of individuals with AD was recently shown to contain tau filaments (Stern et al., 2023, supplemental figures). Therefore, it is possible T22 recognized tau filaments in the synapses studied by Colom-Cadena et al. Additional studies are required to identify the molecular species of tau present in synapses in the brains of individuals with AD.
TrueBinding
It would be interesting to investigate anterograde transsynaptic spreading of tau oligomers, and also which particular species of these tau oligomers are present in the postsynaptic terminal. Another important experiment is to characterize these tau oligomers with MC1, a conformation-dependent antibody (epitope within aa 312-322). Structural characterization of these oligomers in synapses of AD and other tauopathies brains will be essential for exploring therapeutic modalities.
University of California, San Francisco
This study by the Spires-Jones group shows accumulation of tau oligomers in pre- and postsynaptic terminals, including in regions with low or no NFT presence. This work provides beautiful and precise quantification of pre- vs. postsynaptic localization, and confirms and extends previous work by us and others supporting synaptic spread of tau pathology using human postmortem samples.
With respect to extracellular vesicle spread, tau oligomers have also been shown on extracellular vesicles (EVs) released from human AD synapses (Miyoshi et al., 2021). These EVs demonstrated seeding activity in tau biosensor cells.
It is also possible that p-tau aggregation is directly driven by synaptic oligomeric Aβ prior to anterograde and retrograde spread across synapses. In a 2016 paper (Bilousova et al., 2016), we used parietal cortex samples from early stage disease (Braak II-III) to quantify p-tau and Aβ by flow cytometry on an individual terminal basis. Aβ-positive synaptosomes showed a more than twofold increase in p-tau relative to the total population in multiple regions. Importantly, early stage samples from hippocampus and entorhinal cortex showed a strong correlation between synaptic Aβ and p-tau levels by flow cytometry.
Taken together with multiple lines of evidence, including the exclusive initial appearance of Aβ in the neocortex before dementia onset, these experiments suggest that a prion-like mechanism of endogenous templated protein corruption may occur in early disease (Walker et al., 2013).
References:
Bilousova T, Miller CA, Poon WW, Vinters HV, Corrada M, Kawas C, Hayden EY, Teplow DB, Glabe C, Albay R 3rd, Cole GM, Teng E, Gylys KH. Synaptic Amyloid-β Oligomers Precede p-Tau and Differentiate High Pathology Control Cases. Am J Pathol. 2016 Jan;186(1):185-98. PubMed.
Miyoshi E, Bilousova T, Melnik M, Fakhrutdinov D, Poon WW, Vinters HV, Miller CA, Corrada M, Kawas C, Bohannan R, Caraway C, Elias C, Maina KN, Campagna JJ, John V, Gylys KH. Exosomal tau with seeding activity is released from Alzheimer's disease synapses, and seeding potential is associated with amyloid beta. Lab Invest. 2021 Dec;101(12):1605-1617. Epub 2021 Aug 30 PubMed.
Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013 Mar 1;70(3):304-10. PubMed.
Make a Comment
To make a comment you must login or register.