Alzheimer’s Disease Linked to Superficial Siderosis. What Does it Mean?
Quick Links
People with Alzheimer’s disease are nearly seven times more likely than other people to harbor superficial siderosis (SS)—a serpentine pattern of blood deposits that snake the brain’s surface, researchers reported in the February 25 Neurology. The blood dregs, which show up on MRI scans, have previously been linked to amyloid-related vascular injury. The new work, led by Frederik Barkhof at VU University Medical Center in Amsterdam, extends the superficial siderosis link to AD, and may provide a useful radiological marker for vascular amyloid pathology that plagues some AD patients.
“This is a really important study,” Berislav Zlokovic at the University of Southern California, Los Angeles, who was not involved in the study, told Alzforum. “It basically tells us that the blood-brain barrier in these folks is damaged, and these lesions could play an important role in dementia and neurodegeneration.”
Cortical superficial siderosis (SS) is characterized by deposits of hemosiderin, an iron-containing breakdown product of blood, that line the outermost surface of the cortex (see image below). The deposits accumulate in the subpial space, just underneath the delicate pial membrane that enshrouds the brain. Chronic bleeding from within the cortex, or from the subarachnoid space just outside of it, are possible sources of the leakage.
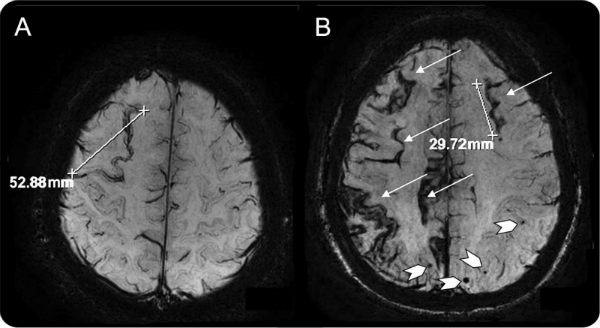
An MRI scan shows tracks of hemosiderin (arrows, right), a byproduct of blood, tracing the cortical surface in the brain of a person with Alzheimer’s disease. The condition often occurs in association with microbleeds (chevrons, right). [Image courtesy of Frederick Barkhof. From “Prevalence of cortical superficial siderosis in a memory clinic population,” published in Neurology 82, February 25, 2014, pp. 698-704, © 2014 American Academy of Neurology. Used with permission of Lippincot Williams and Wilkins]
To tease out the connection between AD and SS, first author Hazel Zonneveld and colleagues analyzed MRI scans from 809 patients in the Amsterdam Dementia Cohort. The researchers identified SS in 17 patients (2.1 percent of the total), as compared with 0.7 percent found in the general population from another study (see Vernooij et al., 2009). Considering patients by diagnosis revealed that at 4.8 percent, people with AD had the highest prevalence of SS. It hovered around 2 percent in those with mild cognitive impairment or other forms of dementia, while people with subjective memory complaints or other disorders appeared SS-free.
Interestingly, nearly 6 percent of people with two copies of the ApoE4 allele had SS, whereas only 1 percent of others did. Zlokovic said this result makes sense given recent evidence from his and other labs showing that ApoE4 compromises the blood-brain barrier, and prevents clearance of Ab from the brain vasculature (see May 2012 news story and April 2013 news story).
One potential trigger of the bleeding is cerebral amyloid angiopathy (CAA), a condition in which vascular deposits of Aβ damage blood vessels in the brain. CAA is suspected to cause the tiny microbleeds found in the brains of some people with AD, and may even trigger larger intracerebral hemorrhages. Unfortunately, CAA can only be confirmed by directly examining brain tissue through biopsy or autopsy, as PET imaging methods aren’t sensitive enough to distinguish between vascular and parenchymal Aβ. In 2008, two small biopsy studies revealed that people with superficial siderosis also turned out to have CAA, hinting that perhaps the amyloid-ravaged blood vessels could have caused the more extensive blood leaks (see Linn et al., 2008, and Feldman et al., 2008).
A larger study in 2010 found that 60.5 percent of people with confirmed CAA also had SS, as opposed to zero cases of siderosis in patients who had suffered an intracerebral hemorrhage but who had no signs of CAA (see Linn et al., 2010). Further studies strengthened ties between CAA and SS (see Charidimou et al., 2013), and a recent study showed that people with cognitive impairment had higher rates of SS than those of the general population (see Wollenweber et al., 2014). However, none have definitively shown that people with AD are more likely to suffer siderosis.
The distinction between CAA and AD is important, Barkhof wrote to Alzforum. “In AD, the distribution of amyloid differs from that in sporadic CAA,” he wrote. He also noted that Aβ-targeting immunotherapies have been known to cause vascular side effects, so understanding the relationship between SS and AD may be important when considering treatment.
“This is a good study and raises some interesting questions,” said Jennifer Linn at University Hospital Munich in Germany. “It’s unclear if SS itself contributes to cognitive impairment in AD, or whether it’s just a marker of co-existing CAA. That’s a question that is absolutely unsolved.”
Zlokovic is convinced that vascular abnormalities such as microbleeds and SS contribute to disease. “I believe that these features are not just an epiphenomenon,” he said. “On the contrary, they may influence or accelerate the development of chronic neurological disorders.”
Because other vascular abnormalities, such as microbleeds, commonly occur in AD patients, Zonneveld and colleagues looked at the MRI scans for associations between SS and other signs of vessel damage. They found that the brains of people with SS were more likely than others to show white-matter hyperintensities, a possible sign of vascular damage. Ninety-four percent of patients with SS had these microbleeds, compared with 24 percent of patients in the entire cohort. On average, patients with SS had 12 times the number of microbleeds of those without SS. Hinting at a possible relationship between the microbleeds and SS, most of the patients with SS had at least one microbleed in close proximity to the siderosis.
What, if there is one, is the connection between microbleeds and SS? Linn said some researchers think microbleeds may cause SS, but she strongly suspects the two conditions are caused by leakage from different blood vessels. The leptomeningeal vessels crisscross the subarachnoid space—a thin layer filled with cerebrospinal fluid that sits just outside the subpial membrane that covers the cortex. When these vessels leak, blood enters the subarachnoid space. Based on preliminary results from ongoing longitudinal MRI studies in her lab, Linn said that subarachnoid hemosiderin then crosses the subpial membrane, which leads to superficial siderosis. Microbleeds, on the other hand, could be more likely caused by leakage from vessels within the cortex, Linn said. Amyloid deposition can compromise both leptomeningeal and cortical vessels, she said, so clusters of amyloid in the same region of the brain could make microbleeds and SS neighbors.
The strong connection between SS and AD, and to a lesser extent other cognitive impairments, is interesting, said Roy Weller of University of Southampton, England, yet many questions remain unanswered. Postmortem analysis of brains with SS would be needed to definitively link amyloid angiopathy with SS, he said. “Until that’s done, it’s going to be difficult to say exactly what SS represents."
If the link between amyloid angiopathy and SS is eventually solidified, Weller said, radiology (rather than biopsy or autopsy) could possibly be used to assess CAA in AD patients. “As long as you know what the radiology indicates pathologically, you can look at far more patients,” Weller said, “and you can look at them when they’re alive.”—Jessica Shugart
References
News Citations
- ApoE4 Makes Blood Vessels Leak, Could Kick Off Brain Damage
- ApoE Does Not Bind Aβ, Competes for Clearance
Paper Citations
- Vernooij MW, Ikram MA, Hofman A, Krestin GP, Breteler MM, van der Lugt A. Superficial siderosis in the general population. Neurology. 2009 Jul 21;73(3):202-5. PubMed.
- Linn J, Herms J, Dichgans M, Brückmann H, Fesl G, Freilinger T, Wiesmann M. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol. 2008 Jan;29(1):184-6. Epub 2007 Oct 18 PubMed.
- Feldman HH, Maia LF, Mackenzie IR, Forster BB, Martzke J, Woolfenden A. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke. 2008 Oct;39(10):2894-7. PubMed.
- Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, van Buchem MA, Bruckmann H, Greenberg SM. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010 Apr 27;74(17):1346-50. PubMed.
- Charidimou A, Jäger RH, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Werring DJ. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2013 Aug 13;81(7):626-32. Epub 2013 Jul 17 PubMed.
- Wollenweber FA, Buerger K, Mueller C, Ertl-Wagner B, Malik R, Dichgans M, Linn J, Opherk C. Prevalence of cortical superficial siderosis in patients with cognitive impairment. J Neurol. 2014 Feb;261(2):277-82. Epub 2013 Nov 13 PubMed.
Further Reading
Papers
- Kantarci K, Gunter JL, Tosakulwong N, Weigand SD, Senjem MS, Petersen RC, Aisen PS, Jagust WJ, Weiner MW, Jack CR, . Focal hemosiderin deposits and β-amyloid load in the ADNI cohort. Alzheimers Dement. 2013 Oct;9(5 Suppl):S116-23. PubMed.
- Na HK, Park JH, Kim JH, Kim HJ, Kim ST, Werring DJ, Seo SW, Na DL. Cortical superficial siderosis: a marker of vascular amyloid in patients with cognitive impairment. Neurology. 2015 Feb 24;84(8):849-55. Epub 2015 Jan 28 PubMed.
Primary Papers
- Zonneveld HI, Goos JD, Wattjes MP, Prins ND, Scheltens P, van der Flier WM, Kuijer JP, Muller M, Barkhof F. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology. 2014 Feb 25;82(8):698-704. Epub 2014 Jan 29 PubMed.
Annotate
To make an annotation you must Login or Register.
