Among AD Mutations, Only ApoE4 Seems to Hobble Microglia
Quick Links
New research adds to the evidence that ApoE regulates microglial health and activity. Researchers led by Tarja Malm at the University of Eastern Finland, Kuopio, examined how several Alzheimer’s risk factors affect microglia generated from human induced pluripotent stem cells. In the October 8 Stem Cell Reports, they describe a new protocol for rapidly generating these microglia. In these cells, the ApoE4 allele suppressed energy production, phagocytosis, and cell migration, while upping release of proinflammatory cytokines. By contrast, familial AD mutations in amyloid precursor protein and presenilin 1 left microglial function largely alone.
- A new protocol quickly generates human microglia from iPS cells.
- Familial AD mutations in APP and PS1 barely affect these cells.
- ApoE4 weakens their phagocytosis and heightens inflammatory responses.
The findings confirm recent reports of the key role ApoE plays in orchestrating microglial responses to disease. They also reinforce the concept that familial and sporadic disease start in different cells, with inflammatory processes more important in the latter. “ApoE4 contributes to AD risk via microglia, while familial AD mutations act on other brain cell types,” Malm noted.
Previous work from Oleg Butovsky at Brigham and Women’s Hospital, Boston, found that ApoE is required for mouse microglia to rev up phagocytosis in response to neurodegeneration (Sep 2017 news). The E4 allele impairs this phagocytic response (May 2019 conference news).
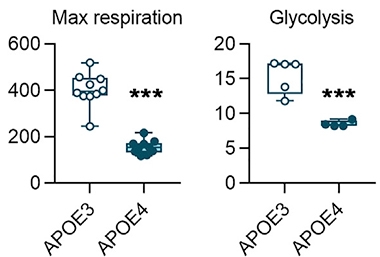
Metabolism Muted. Human ApoE4 microglia have less than half the capacity of their ApoE3 counterparts to produce energy, either by oxidative respiration (left) or glycolysis (right). [Courtesy of Konttinen et al., Stem Cell Reports.]
Few studies have examined what APP and PS1 mutations might do in microglia, although some work suggests that they can alter their inflammatory responses and Aβ uptake (Manocha et al., 2016; Zhao et al., 2017). These mouse studies may not translate to people, however, as research indicates that mouse and human microglia respond differently to disease (Feb 2018 news).
To investigate human cell responses, Malm and colleagues developed a protocol for generating microglia from induced pluripotent stem cells. They tried to mimic the in vivo differentiation of microglia from the yolk sac, first subjecting iPSCs to low-oxygen conditions to generate erythromyeloid progenitors, then adding growth factors to guide development into mature microglia. The protocol took 24 days and yielded 20 microglia for every iPS cell.
Microglia rapidly alter their gene expression when cultured, which complicates their study (Jun 2017 news). Importantly, Malm’s iPSC-derived cells did not express macrophage genes, suggesting the protocol produced a pure microglial population. The cells had the ramified shape typical of human microglia. Their transcriptional profile matched that of other iPS-derived microglia, as well as microglia freshly isolated from human brain (Zhang et al., 2016; Abud et al., 2017).
The method resembles one developed by Mathew Blurton-Jones and colleagues at the University of California, Irvine, though that one did not rely on low oxygen and takes two weeks longer (McQuade et al., 2018).
Julia TCW at the Icahn School of Medicine at Mount Sinai, New York, praised Malm’s protocol, noting that it appears robust and well-validated. “Because it’s simple and fast, it could be more practical than other methods. These induced microglia could be utilized for many assays,” TCW told Alzforum.
For this study, first author Henna Konttinen initially generated iPSC lines from fibroblasts donated by 12 volunteers, then differentiated them into microglia. Two of the donors carried the Swedish mutation in APP, two had the presenilin 1 DE9 deletion, three were homozygous for ApoE4, and the remaining five were from healthy ApoE3/3 controls.
The APP and PS1 mutant microglia behaved like wild-type microglia in most assays. They produced the same amount of Aβ42 as controls and gobbled up the yeast glycan zymosan as well as controls. When stressed, APP and PS1 microglia did behave slightly differently. They migrated faster than wild-type to fill a gap in the cell layer in the culture dish. They released less of several pro-inflammatory cytokines when stimulated with lipopolysaccharide or interferon-γ to mimic inflammation. Their weak inflammatory response suggests that APP and PS1 mutant microglia may have a senescent phenotype and are unable to mount an immune reaction, the authors noted.
“We were surprised that these familial mutations did very little to microglial function,” Malm said. TCW noted that APP and PS1 are barely expressed in microglia under basal conditions.
ApoE4 microglia were a different story. These cells ingested fewer yeast particles than did ApoE3 controls, and they moved more slowly to fill in a void in the cultured cell layer. Upon stimulation with LPS and IFN-γ, E4 microglia ramped up secretion of several proinflammatory cytokines, including IL-6, IL-8, MCP-1, TNFα, and RANTES. Overall, the findings suggest a shift from a phagocytic to an inflammatory phenotype.
The results agree with other recent work. “In general, their findings are consistent with ours,” Li-Huei Tsai at Massachusetts Institute of Technology, Cambridge, wrote to Alzforum. She found sluggish phagocytosis and a heated inflammatory response in two ApoE3/4 induced human microglial lines compared to isogenic ApoE3/3 controls (Jun 2018 news). Likewise, TCW recently reported a higher inflammatory response in E4/4 than E3/3 human microglia (Aug 2019 news). In ongoing work, TCW sees preliminary evidence of poor phagocytosis in ApoE4/4 microglia as well, she told Alzforum.
Curiously, research in a mouse microglial cell line reported the opposite—increased motility and phagocytosis in ApoE4 microglia compared to E3s (Muth et al., 2019). “Our study, together with other recent research, underlines the importance of modeling function using human cells,” Malm noted.
The ApoE4 allele also put a damper on mitochondrial energy production, halving oxygen consumption and ATP production. Anaerobic glycolysis was flat, suggesting overall metabolism was slowed by the E4 allele (see image above).
“The effect of ApoE isoform on microglial metabolism is an interesting aspect of this study,” wrote Jason Ulrich, Washington University in St. Louis, to Alzforum (full comment below). He noted that a previous study reported low microglial oxidative and glycolytic energy production in 5xFAD mice that lacked TREM2 (Aug 2017 news). “Glycolytic activation of microglia could play an important role in the microglial response to AD pathology,” he wrote. “It would be interesting to know how ApoE genotype would influence microglial metabolism in response to stimuli more associated with neurodegenerative disease, such as myelin debris or dying neurons.”—Madolyn Bowman Rogers
References
News Citations
- ApoE and Trem2 Flip a Microglial Switch in Neurodegenerative Disease
- On The Docket at AD/PD: The Many Crimes of ApoE4
- Microglial Transcriptome Hints at Shortcomings of AD Model
- What Makes a Microglia? Tales from the Transcriptome
- In Human Neurons, ApoE4 Promotes Aβ Production and Tau Phosphorylation
- ApoE4 Glia Bungle Lipid Processing, Mess with the Matrisome
- Without TREM2, Microglia Run Out of Gas
Mutations Citations
Research Models Citations
Paper Citations
- Manocha GD, Floden AM, Rausch K, Kulas JA, McGregor BA, Rojanathammanee L, Puig KR, Puig KL, Karki S, Nichols MR, Darland DC, Porter JE, Combs CK. APP Regulates Microglial Phenotype in a Mouse Model of Alzheimer's Disease. J Neurosci. 2016 Aug 10;36(32):8471-86. PubMed.
- Zhao Y, Li X, Huang T, Jiang LL, Tan Z, Zhang M, Cheng IH, Wang X, Bu G, Zhang YW, Wang Q, Xu H. Intracellular trafficking of TREM2 is regulated by presenilin 1. Exp Mol Med. 2017 Dec 1;49(12):e405. PubMed.
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016 Jan 6;89(1):37-53. Epub 2015 Dec 10 PubMed.
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. PubMed.
- McQuade A, Coburn M, Tu CH, Hasselmann J, Davtyan H, Blurton-Jones M. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener. 2018 Dec 22;13(1):67. PubMed.
- Muth C, Hartmann A, Sepulveda-Falla D, Glatzel M, Krasemann S. Phagocytosis of Apoptotic Cells Is Specifically Upregulated in ApoE4 Expressing Microglia in vitro. Front Cell Neurosci. 2019;13:181. Epub 2019 May 3 PubMed.
Further Reading
Primary Papers
- Konttinen H, Cabral-da-Silva ME, Ohtonen S, Wojciechowski S, Shakirzyanova A, Caligola S, Giugno R, Ishchenko Y, Hernández D, Fazaludeen MF, Eamen S, Budia MG, Fagerlund I, Scoyni F, Korhonen P, Huber N, Haapasalo A, Hewitt AW, Vickers J, Smith GC, Oksanen M, Graff C, Kanninen KM, Lehtonen S, Propson N, Schwartz MP, Pébay A, Koistinaho J, Ooi L, Malm T. PSEN1ΔE9, APPswe, and APOE4 Confer Disparate Phenotypes in Human iPSC-Derived Microglia. Stem Cell Reports. 2019 Oct 8;13(4):669-683. Epub 2019 Sep 12 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Boston University School of Medicine
Konttinen et al. have developed another microglia protocol based on human iPSC-derived primitive (but not definitive) erythromyeloid progenitors induced by hypoxia. The protocol is similar to but slightly simpler than that described by Abud and colleagues (Abud et al., 2017), resulting in a similar transcriptomic profile of microglia. Konttinen et al. have differentiated 16 lines, indicating the method is robust.
As it’s known from primary human brain transcriptomic data (Zhang et al., 2016), APP and PSEN1 are not specifically expressed in microglia but are in neurons and oligodendrocytes. Thus, it is expected that those genotypes would not affect many microglial assays. However, APOE shows glial-specific expression and its protein levels differ by APOE genotype both in astrocytes and microglia (TCW et al., 2019). Therefore, it could influence multiple glial functions including phagocytosis.
I would like to emphasize that human and mouse microglia may behave quite differently based on the authors’ finding that the APOE4 genotype in human microglia impairs migration and phagocytosis, while expressing the human APOE4 gene in mouse microglia increases migration (the opposite result for the same scratch assay/wound density measurement) and enhances phagocytosis (Muth et al., 2019). We would further need to compare human and mouse microglia side by side to figure out what causes these differences.
An interesting functional finding of this paper in terms of APOE4 in human microglia is that APOE4 did not affect calcium transients but did lower oxygen consumption rate similar to LPS stimulation. On the assumption that there is no uptake on external APOE-lipids, it will be interesting to investigate what causes mitochondrial dysfunction by APOE4 genotype.
References:
Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. PubMed.
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016 Jan 6;89(1):37-53. Epub 2015 Dec 10 PubMed.
Tcw J, Qian L, Pipalia NH, Chao MJ, Liang SA, Shi Y, Jain BR, Bertelsen SE, Kapoor M, Marcora E, Sikora E, Andrews EJ, Martini AC, Karch CM, Head E, Holtzman DM, Zhang B, Wang M, Maxfield FR, Poon WW, Goate AM. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022 Jun 23;185(13):2213-2233.e25. PubMed. BioRxiv.
Muth C, Hartmann A, Sepulveda-Falla D, Glatzel M, Krasemann S. Phagocytosis of Apoptotic Cells Is Specifically Upregulated in ApoE4 Expressing Microglia in vitro. Front Cell Neurosci. 2019;13:181. Epub 2019 May 3 PubMed.
Washington University
Konttinen and coworkers add to the rapidly growing body of work examining the effect of AD risk factors using induced human microglia derived from iPSCs. The observed reductions in phagocytosis and increased inflammatory cytokine production in APOE4 microglia echo the recent report from Lin, Seo, and colleagues that found APOE4 reduced Aβ42 uptake and increased inflammatory gene expression pathways (Lin et al., 2018). Similar enhancements of inflammatory signaling by APOE4 have also previously been described using primary microglia obtained from APOE3 or APOE4 targeted replacement mice (Vitek et al., 2009).
The effect of APOE isoform on microglial metabolism is an interesting aspect of this study. Ulland, Song and colleagues reported TREM2 knockout reduced glycolysis and mitochondrial mass in microglia in 5xFAD mice, indicating that glycolytic activation of microglia could play an important role in the microglial response to AD pathology (Ulland et al., 2017). It would be interesting to know how APOE genotype would influence the microglial metabolism in response to stimuli more associated with neurodegenerative disease, such as myelin debris or dead/dying neurons.
References:
Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, Rueda R, Kritskiy O, Abdurrob F, Peng Z, Milo B, Yu CJ, Elmsaouri S, Dey D, Ko T, Yankner BA, Tsai LH. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018 Jun 27;98(6):1141-1154.e7. Epub 2018 May 31 PubMed.
Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009 Sep;30(9):1350-60. PubMed.
Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer's Disease. Cell. 2017 Aug 10;170(4):649-663.e13. PubMed.
University of Arkansas for Medical Sciences
In their article, Konttinen et al. write: "[E]xact mechanisms underlying human APOE4-induced inflammatory phenotype in AD microglia remain incompletely defined." Their study and several other recent publications suggest a strong possibility of a cell-autonomous—potentially intracellular—mechanism. Here is one worth considering: Parcon et al., 2018, "Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs."
References:
Parcon PA, Balasubramaniam M, Ayyadevara S, Jones RA, Liu L, Shmookler Reis RJ, Barger SW, Mrak RE, Griffin WS. Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs. Alzheimers Dement. 2018 Feb;14(2):230-242. Epub 2017 Sep 22 PubMed.
Make a Comment
To make a comment you must login or register.