Antibodies Boost Microglial Appetite for Tau
Quick Links
The brain’s resident immune cells have a taste for tau, and anti-tau antibodies act like the secret sauce. According to a June 9 study in Scientific Reports, microglia dine on pathogenic forms of tau released from brain tissue affected by Alzheimer’s disease. The cells internalized tau more readily when anti-tau antibodies were added to the mix, and the researchers found that the Fc (stem) portion of the antibody was required for this enhancement. While in vivo experiments are still lacking, the findings hint that microglia play a role in clearing toxic tau from the brain, and that the success of future tau immunotherapies may hinge upon improving the action of these brain sentinels.
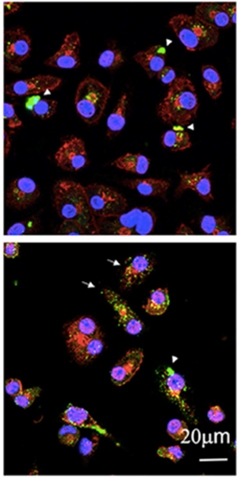
Tummy Full of Tau.
Microglia internalize tau (green) from AD brain samples. Both AT8 (top panel) and MC1 (bottom panel) antibodies recognize tau in the microglia (red, wheat germ agglutinin; blue, nuclei). [Courtesy of Luo et al., Scientific Reports 2015.]
“Our data strongly suggest that if tau exists in the extracellular space, it will be taken up and degraded by microglia, and antibodies could enhance the process,” said Steven Paul of Weill Cornell Medical College in New York, the paper’s senior author.
In healthy neurons, tau stabilizes the microtubules that ferry important proteins between the cell body and the axon’s outer reaches. When hyperphosphorylated, tau ditches microtubules and forms intracellular tangles instead. Researchers have found that in addition to intracellular aggregates, tau can exit neurons and enter neighboring ones, thus spreading pathology throughout the brain (see Mar 2009 conference news; Jun 2009 news).
Efforts to target pathogenic forms of tau using active and passive immunizations have exploded. A handful of therapies are in, or about to enter, Phase 1 trials, while a plethora of others are in preclinical development (see Pedersen and Sigurdsson, 2015). In preclinical studies, anti-tau antibodies reduced tauopathy in transgenic mice and improved cognitive function (see Boutajangout et al., 2011; Chai et al., 2011; Sep 2013 news).
However, the way these antibodies work their magic is still unknown. They could enter neurons and bind to intracellular tau, shuttling the protein into lysosomes and/or preventing it from spreading to other cells (see Congdon et al., 2013; Gu et al., 2013; Collin et al., 2014). Alternatively, the antibodies could latch onto extracellular tau. Using their Fc receptors, microglia would then internalize these tau-antibody complexes and digest them. Either or both mechanisms could occur in the AD brain, and may vary depending upon the antibody and the form of tau that it targets.
First author Wenjie Luo and colleagues tested whether microglia ingested tau, and if it did, whether antibodies could influence the process. They started by isolating sarkosyl-insoluble tau from postmortem AD brain samples that were riddled with tau tangles. This fraction of tau was highly enriched with paired helical filament (PHF)-tau, which is thought to represent a pathogenic species. Then the researchers incubated this insoluble tau with primary microglia derived from neonatal mouse brains. After two days, the researchers found that only 20 percent of total tau and 5 percent of hyperphosphorylated tau remained in the media, whereas most of the tau remained when microglia were absent. Using confocal microscopy, the researchers observed hyperphosphorylated tau within discrete puncta inside the microglia, as well as some large tau aggregates attached to their cell membranes. They saw a dramatic rise in intracellular tau within the first two hours of incubation, followed by a steep decline. They concluded that microglia internalized and then degraded tau derived from AD brain tissue.
The researchers next measured whether microglia would internalize tau released from brain slices. They added microglia to cultures of thawed brain slices from P301S transgenic mice, which harbor an abundance of tau tangles. The researchers found that the tau concentration in the slice culture medium initially rose, and then plummeted as microglia internalized the protein. In cultures without microglia, tau levels held steady after the initial bump. Tau levels also remained unchanged when researchers added media from microglial cultures to the slices, indicating that enzymes secreted by microglia did not degrade tau. The researchers observed a similar microglia-dependent reduction in tau derived from postmortem frontal cortex slice cultures of advanced-stage AD patients, and also found that microglia internalized Aβ40 and Aβ42 from those slices in addition to tau.
Interestingly, microglia also took a bite out of neurofibrillary tangles in the slice cultures, as measured by immunohistochemistry. How the microglia access the intracellular tangles is unclear, Paul said. It is important to note that because the slices measured only 10 microns thick, few cells survived slicing, and intracellular proteins (including tangles) could leak out into the medium. Paul would not speculate on whether microglia could somehow access the intracellular compartments. He said the most important aspect of the findings was that microglia readily internalize a variety of pathogenic tau species.
Next the researchers measured the effect of anti-tau antibodies on microglial internalization of tau. They mixed a fluorescently labeled AT8 antibody—which recognizes hyperphosphorylated tau—with P301S brain slices, washed away any unbound antibodies, and then added microglia. One hour later, they observed an abundant fluorescent signal inside the microglia, indicating that the cells had consumed the tau/antibody complexes. Next, the researchers mixed MC1—another anti-tau antibody that recognizes a pathological conformation of tau—with sarkosyl-insoluble tau isolated from AD brains. They found that microglia took up tau bound by MC1 more efficiently than tau alone, but that other IgG antibodies did not enhance uptake. This enhancement vanished when the researchers instead added a Fab fragment, which lacks the Fc effector portion of the antibody, to the tau prep.
“Their findings are as expected based on known functions of microglia, and further confirm the feasibility of tau immunotherapy,” wrote Einar Sigurdsson of New York University.
Paul proposed that using anti-tau antibodies could rev up flagging microglial clearance responses in the AD brain. Microglial functions, including phagocytosis, are known to decline with age, and variants in the AD risk genes TREM2 and CD33 exacerbate these deficits, Paul said (see July 2014 Webinar; Aug 2013 news; Griciuc et al., 2013). He proposed that defective microglia fail to clear both amyloid and tau in two parallel pathways that ultimately lead to AD. Paul proposed that antibodies that engage microglial Fc receptors could “supercharge” the cells’ cleanup capacities.
Takami Tomiyama of Osaka City University in Japan commented that the findings indicate that antibodies without Fc regions could have lower tau clearance rates in vivo, despite the fact that these smaller antibody fragments enter the brain more readily than their full-sized cousins. “Moreover, this notion would prompt us to select antibodies with a higher binding affinity to Fc receptors during the development of therapeutic antibodies,” he wrote.
Paul’s findings indicate that microglial internalization is one possible mechanism of antibody-mediated tau clearance, commented Yona Levites of the University of Florida in Gainesville. However, it is not yet possible to rule out the possibility that other non-microglial mechanisms, such as entrance of the antibodies into neurons, contribute to therapeutic effects in vivo. Levites added that emerging data from her lab and others suggests that Fab and single-chain variable fragment (scFv) antibodies clear tau in mouse models just as well as those with Fc portions attached.
Martin Citron of UCB Pharma in Brussels referenced similar reports, pointing out that researchers from Genentech/AC Immune recently presented findings at the AD/PD conference in Nice suggesting that antibodies with or without Fc regions cleared tau equally well. He added that it will be important to understand why, in the case of Paul’s antibody, the Fc portion was needed. “The paper does not address whether the Fab fragment is less active due to loss of effector function or loss of the avidity benefit of an antibody,” he wrote. “Clearly, more work is needed to fully understand the mechanisms of tau clearance and tau immunotherapy in cell systems and in vivo.”
Paul said his lab is currently investigating whether microglial function or mutations in AD risk genes such as TREM2 influence the efficacy of anti-tau antibodies in mouse models.—Jessica Shugart
References
News Citations
- Keystone: Tau, Huntingtin—Do Prion-like Properties Play a Role in Disease?
- Traveling Tau—A New Paradigm for Tau- and Other Proteinopathies?
- Antibodies Stop Toxic Tau in Its Extracellular Tracks
- Protective Microglial Gene Variant Promotes Phagocytosis
Antibody Citations
Alzpedia Citations
Webinar Citations
Paper Citations
- Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer's disease. Trends Mol Med. 2015 Jun;21(6):394-402. Epub 2015 Apr 3 PubMed.
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011 Aug;118(4):658-67. PubMed.
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O'Neill MJ, Hutton ML, Citron M. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011 Sep 30;286(39):34457-67. PubMed.
- Congdon EE, Gu J, Sait HB, Sigurdsson EM. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013 Dec 6;288(49):35452-65. Epub 2013 Oct 25 PubMed.
- Gu J, Congdon EE, Sigurdsson EM. Two novel tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce tau pathology. J Biol Chem. 2013 Nov 15;288(46):33081-95. PubMed.
- Collin L, Bohrmann B, Göpfert U, Oroszlan-Szovik K, Ozmen L, Grüninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain. 2014 Oct;137(Pt 10):2834-46. Epub 2014 Jul 31 PubMed.
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013 May 22;78(4):631-43. PubMed.
Other Citations
Further Reading
Papers
- Gerson JE, Castillo-Carranza DL, Kayed R. Advances in therapeutics for neurodegenerative tauopathies: moving toward the specific targeting of the most toxic tau species. ACS Chem Neurosci. 2014 Sep 17;5(9):752-69. Epub 2014 Aug 8 PubMed.
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015 Jun;16(6):358-72. PubMed.
Primary Papers
- Luo W, Liu W, Hu X, Hanna M, Caravaca A, Paul SM. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci Rep. 2015 Jun 9;5:11161. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCB Pharma Belgium
In this Scientific Reports publication, Steve Paul and colleagues demonstrate microglial degradation of pathological tau in a number of in vitro and ex vivo paradigms, including incubation of human brain sections with primary mouse microglia. They also show enhancement of this degradation by MC1, an antibody binding to pathological tau, and suggest that this effect is Fc-mediated, because the MC1 Fab fragment is not active in the assay. The paper does not address whether the Fab fragment is less active due to loss of effector function or loss of the avidity benefit of an antibody.
Overall, these are exciting new data reminiscent of and in line with work on microglial-mediated Aβ plaque clearance from 2000 on from, e.g., Bard et al. (Bard et al., 2000), Steve’s group, and others.
It now remains to be shown whether this mechanism plays out for tau in vivo. This is not straightforward: Passive immunization data presented by the Genentech/AC Immune team at the last ADPD meeting showed equal efficacy of full effector or effectorless antibodies in a P301L transgenic model (Ayalon et al., abstract 1499), suggesting that, at least for their antibody, induction of microglial clearance is not a critical contributor to efficacy.
Clearly, more work is needed to fully understand the mechanisms of tau clearance and tau immunotherapy in cell systems and in vivo.
References:
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000 Aug;6(8):916-9. PubMed.
Osaka City University Graduate School of Medicine
While many studies have shown that active and passive tau immunization successfully attenuate tau pathology in tauopathy model mice, the underlying mechanism is poorly understood. Recent evidence suggests that pathological tau species secreted from affected neurons are taken up by the connecting neurons in which internalized tau aggregates promote de novo aggregation by endogenous soluble tau, which explains how tau pathology spreads in the brain. This hypothesis implies that anti-tau antibodies attenuate tau pathology by sequestering extracellular tau and thereby preventing tau pathology propagation. In the present study, Luo et al. clearly demonstrate that brain microglia also participate in the clearance of extracellular tau. Mouse primary microglia were shown to rapidly internalize and degrade tau aggregates derived from AD brain. Furthermore, an anti-tau monoclonal antibody, but not its Fab fragment, facilitated this degradation, suggesting that microglia internalize extracellular tau not only by phagocytosis but also by Fc receptor-mediated endocytosis of immune complexes. A similar phenomenon was previously observed with Aβ.
Thus, the present study gives the opportunity to investigate the clearance mechanism for extracellular tau in comparison with that for Aβ. Four possible mechanisms have been proposed for Aβ clearance: 1) degradation by membrane-bound or extracellular enzymes such as neprilysin and insulin-degrading enzyme, 2) internalization into neuronal/glial cells and degradation in the lysosomes, 3) receptor-mediated transcytosis at the blood-brain barrier and efflux into the blood, and 4) efflux into the CSF or lymph nodes through the perivascular lymphatic drainage. The present study indicates that the first mechanism is unlikely for tau, since conditioned media of microglia contained little tau-degrading activity. The second mechanism is the most plausible in tau clearance. Microglia were shown to internalize and degrade extracellular tau, and antibodies enhanced this process. The latter finding cautions that Fc-truncated versions of IgG such as single-chain Fv might have lower clearance activity against extracellular tau despite their higher efficacy to enter the brain. Moreover, this notion would prompt us to select antibodies with a higher binding affinity to Fc receptors during the development of therapeutic antibodies. Notably, when microglial cells were cultured for 24 hours on unfixed frozen brain sections prepared from tauopathy mice, AT8-positive intracellular tau aggregates (the authors assumed these aggregates were NFTs, but strictly speaking, NFTs should be identified by silver staining) in the sections were significantly reduced. It is surprising that such a rapid elimination (within 24 hours) of intracellular tau aggregates could be achieved only by removing extracellular tau. This finding suggests that intracellular tau is in dynamic equilibrium with extracellular tau. As for the third and fourth mechanisms, their involvement in tau clearance is unclear.
Several studies have shown that antibodies injected into model mice can eliminate pre-existing intracellular tau aggregates, including NFTs. This mechanism is largely unknown. Antibodies may penetrate into cells passing through the plasma membrane and clear tau aggregates in the cytoplasm. Alternatively, there may be a dynamic equilibrium between intracellular and extracellular tau, as mentioned above. Antibodies may shift this equilibrium to induce tau efflux from cells by removing extracellular tau, leading to the eventual elimination of intracellular NFTs. It has been proposed that some misfolded protein pathology spreads in the brain via exosome release from affected cells or tunneling nanotube formation between cells. Whether these mechanisms also contribute to tau pathology propagation, and whether antibodies have any function against them, might be an issue in tau immunotherapy research.
�
Recently, immunotherapy has emerged as a promising approach to target tau, but many mechanistic questions remain regarding how such immunotherapy works. Some of these questions pertaining to mechanism of action of immunotherapy are tightly linked to the potential relative contribution of mechanisms that underlie induction and spread of tau pathology. “Spread” of tau inclusion pathology may result from a combination of mechanisms that includes cell autonomous intrinsic disruption of proteostasis and non-cell autonomous mechanisms — seeding from extracellular tau and induction of a toxic environment induced either by extracellular tau acting as an inflammogen, or by induction of a generalized toxic environment that disrupts proteostasis. Strong data have emerged that antibodies may reduce tau pathology by targeting extracellular tau seeds or by binding, and directly target intracellular tau. It is unclear whether effector function is beneficial for antibodies’ efficacy in vivo. Given these data and the uncertainties regarding the mechanisms of induction and spread of tau pathology in human brain and mouse models of human tauopathies, it is challenging to definitively determine antibody mechanism of action in the disease modification.
The findings in this new paper by Luo et al. are very intriguing, clearly pointing to the fact that antibody presence facilitates microglia-mediated reduction in the levels of pathological tau. It is possible that one of the mechanisms by which passive and active immunization works involves stimulating tau phagocytosis by microglia. However, one has to be cautious in speculating that full antibody presence is essential for potential therapeutic approach in vivo, given the recent findings that Fab fragments (Genentech) as well as scFvs (Levites) are efficient in reducing tau pathology, at least in transgenic mouse models.
In conclusion, more studies have to be done looking at various phospho-tau and conformation-specific tau binding in order to rule out the main mechanism by which tau immunotherapy works.
Prothena Biosciences
This manuscript provides support for the classical concept that microglia cells perform an important homoeostatic activity in CNS. In the context of anti-tau immunotherapy, the findings strengthen the hypothesis that certain antibodies may reduce intracellular tau pathology by acting extracellularly, through neutralization and opsonization of toxic misfolded/aggregated tau species, which may then be cleared by phagocytosis. Therefore, it is conceivable that antibody candidates in development might benefit from retaining their effector function.
The concept that "epitope matters” most likely applies to tau immunotherapy, as it does for other misfolded protein targets in CNS.
Voyager Therapeutics
Thanks to Jessica Shugart for writing a nice summary of our paper and for Martin Citron's thoughtful comments. I'd like to underscore that we are not stating that anti-tau monoclonal antibodies work to reduce tau pathology by an effector-mediated microglial clearance mechanism. What we observed is that for this specific antibody (MC1), using in vitro/ex vivo brain section assays, we saw a stimulation of tau uptake/degradation by the antibody that we did not observe for the Fab. It will take considerably more work to know whether effector function plays any role, good or bad, in the now widely reproduced in vivo effects of anti-tau monoclonal antibodies, i.e., in reducing tau pathology in mouse tauopathy models.
To this day, there are similar debates, and even contradictory data, about anti-Aβ/amyloid monoclonal antibodies. Do they require effector function or not ? My guess is that it is clearly a function to be enhanced, reduced, or potentially eliminated for optimizing tau monoclonal antibodies for therapeutic purposes, but exactly what to do (i.e., what is the optimal antibody?) will require much more work and may not be completey clear until we generate some clinical data in AD/FTD patients.
Our work does highlight a potential role for microglia in clearing extracellular tau species and adds to a growing body of literature suggesting a critical role for microglia in AD pathogenesis. We would caution again, however, that we will need in vivo data to substantiate the hypothesis that microglia function normally to reduce the spread or pathogenicity of tau (several experiments are underway). We are using these assays to also screen for compounds that might enhance microglial-mediated degradation of tau and other pathological/misfolded proteins that clearly play a role in neurodegenerative disorders.
Make a Comment
To make a comment you must login or register.