Are ALS Dipeptide Repeat Ribbons Entangling Proteasomes?
Quick Links
In the past year, cryoelectron microscopy has enabled scientists to crank out molecular structures of tau filaments, Aβ fibrils, and huntingtin aggregates. Now researchers led by Wolfgang Baumeister, Max Planck Institute of Biochemistry, Martinsried, Germany, post another catch in the neurodegenerative arena. In the February 22 Cell, they unveil the shape of aggregates of poly-glycine-alanine, a peptide generated in amyotrophic lateral sclerosis and frontotemporal dementia. They used a version of cryoEM called cryoelectron tomography (a.k.a. cryoET) to peer at aggregates inside rat neurons. They report a dense network of twisted poly-GA ribbons studded with proteasomes, many appearing stuck in the process of chopping up unwanted protein.
- In neurons, ALS/FTD poly-Gly-Ala peptides aggregate into a dense network of twisted ribbons.
- The ribbons sequester a large fraction of the cells’ proteasomes.
- Many trapped proteasomes are frozen in a catalytic transition state.
“This is a welcome paper. There hasn’t been enough attention directed at the proteasome in the ALS field,” said Teepu Siddique, Northwestern University, Chicago. Yun-Ru (Ruby) Chen of Academia Sinica in Taipei, Taiwan, was also enthusiastic, noting the results align with her observations that poly-Gly-Ala (poly-GA) form ribbons in vitro (Chang et al., 2016). “The study will have great impact in the field,” she said. Other commentators questioned the relevance of the findings given that poly-GA’s role in amyotrophic lateral sclerosis/frontotemporal dementia (ALS/FTD) pathogenesis remains uncertain.
The most common genetic cause of ALS/FTD, expanded GGGGCC repeats in the C9ORF72 gene generate five different dipeptide repeat proteins within neurons. No clear correlation has yet been found between poly-GA inclusions and neurodegeneration, and poly-GR/PR peptides are considered more toxic (Freibaum and Taylor, 2017). Even so, poly-GA is most commonly found in inclusions from patient brains (Mckenzie et al., 2015; Mori et al., 2013).
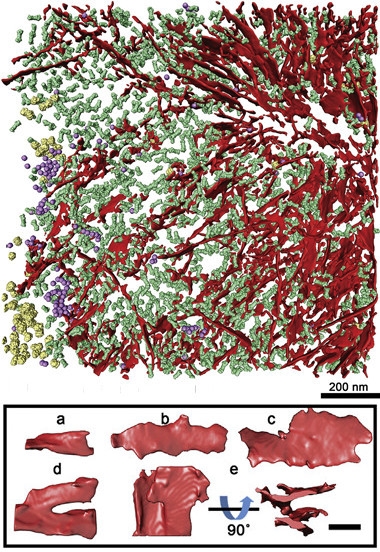
Proteasome trap.
CryoET (top) shows poly-GA ribbons (red) aggregating with proteasomes (green), ribosomes (yellow), and TRIC chaperonins (purple). Close-ups of the ribbons (below) reveal a diversity of shapes. [Courtesy of Cell, Guo et al.]
To get a better look, first author Qiang Guo turned to cryoelectron tomography. While cryoET offers only nanometer resolution, not the atomic-level detail of X-ray crystallography, it works on proteins inside cells (Oikonomu and Jensen, 2017). Baumeister pioneered the approach. “This work is state-of-the-art,” said Sjors Scheres, MRC Laboratory of Molecular Biology, Cambridge, England, U.K., who solved the structure of tau fibrils from human brain with cryoEM (Jul 2017 news).
Guo transduced rat primary neurons with a construct encoding green fluorescent protein (GFP) attached to a string of 175 GA repeats. After five days, it had produced inclusions similar in size and poly-GA content to those found in C9ORF72 patient tissue (May et al., 2014). Guo flash-froze the neurons, then used an ion beam to carve out a 100–200 nm sliver containing a green aggregate visible under the light microscope. “Whole cells are too thick for the electron microscope, so we needed to thin them down,” explained co-author Rubén Fernández-Busnadiego. He placed slivers into the electron microscope and captured images at different angles by tilting the grid. Feeding the data into a computer, he obtained three-dimensional pictures (see above).
The authors were surprised by how different the aggregates looked compared with the mutant huntingtin aggregates they had recently detected using the same technique (Bäuerlein et al., 2017). “Instead of cylindrical fibrils, we saw planar, helically twisted ribbons,” said Fernández-Busnadiego. The ribbons varied in size, but were roughly 14 nm thick, up to 1 μm long, and 20 to 80 nm wide. They were densely packed, often bifurcating and merging with neighboring ribbons. The structures are similar to those described by Chen, who examined 15-repeat poly-GA peptides in vitro using transmission electron and atomic force microscopy. “We first saw fibrils forming, and then the thin ribbons,” she said.
The authors also spotted huge numbers of tiny tubes. In cross-section, they looked like 10 nm rings nestled between the poly-GA ribbons. To sharpen the view, they digitally cut out a few and aligned them to each other, generating a common structure. Reiterating this process to find the most robust structure, they realized—to their amazement—that they were staring at 26S proteasomes.
This is consistent with previous reports of proteasomes in ALS pathology (Aug 2011 news; Gorrie et al., 2014; May et al., 2014). “The results vindicate our initial identification of the causative role of the UPS pathway in ALS and ALS/FTD,” said Siddique. Still, the researchers were struck to count a 30-fold higher concentration of proteasomes in aggregates than other parts of the cell. “It’s a huge accumulation, yet the expression levels of proteasome proteins didn’t change,” said Fernández-Busnadiego, “The aggregates are sucking up the proteasomes like a sponge.”
The researchers also found 20 nm complexes corresponding to the TRiC/CCT chaperonin inside the aggregates, though these were not enriched, and saw ribosomes in the periphery of the aggregates.
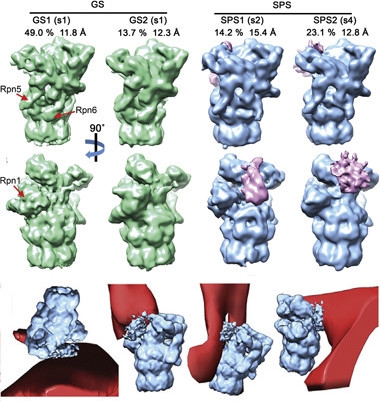
Choking proteasomes?
A majority of aggregate-associated proteasomes are in a quiescent ground state (green), 37 percent are in a substrate-processing state (blue), sporting attached regulatory factors (pink), and touching poly-GA ribbons (red). [Courtesy of Cell, Guo et al.]
Guo noticed that many of the poly-GA-associated proteasomes had bound one or two 19S regulatory particles—protein complexes that recognize, unfold, and translocate substrates into the proteasome core. The authors sorted the proteasomes on that basis, and then averaged the structural information to improve resolution, as they had done for the ribbons. They identified inactive proteasomes and others processing substrates (image at right). Interestingly, aggregates bound a large fraction of the cell’s proteasomes, and 37 percent of them were active. Normally only 20 percent of proteasomes are active at any one time. Moreover, almost a quarter of the poly-GA-bound proteasomes struck a “transient pose.” This ephemeral conformation, in which the substrate is stuck just moving into the proteolytic chamber of the proteasomes, was discovered only recently, when researchers powered proteasomes with a non-hydrolyzable form of ATP to catch them in flagrante (Wehmer et al., 2017). “They seem to be trying to degrade poly-GA, but get stuck,” said Fernández-Busnadiego. Indeed, the number of proteasomes in this state peaked near poly-GA ribbons.
What does this mean for ALS/FTD? Researchers hotly debate which repeat RNAs and proteins generated by C9ORF72 mutations are responsible for pathogenesis. Co-author Dieter Edbauer, German Center for Neurodegenerative Diseases, Munich, thinks different dipeptides play different roles in pathology, and considers poly-GA an important contributor. He told Alzforum that the group found proteasomes in 20 percent of poly-GA-positive inclusions in cortical neurons isolated from ALS patients postmortem, though that bit did not make it into the paper. “We tried poly-GR, but couldn’t see anything,” he said. CryoET sample preparation requires aggregates to be visible in the light microscope so that they can be sectioned out of the cell for subsequent EM. Poly-GA aggregates readily obliged, but poly-GR did not form visible aggregates. He also tried using induced pluripotent stem cells from patients, but these cells formed no suitable aggregates.
Several researchers wondered whether cryoET could be applied to whole tissue from people, or at least mice. Edbauer said high-pressure freezing techniques might help make this possible. “But that is going to take at least five years,” he predicted.
Can the results guide structure-based drug discovery? “The structure we have is not good enough yet. We need better resolution to find ligands,” said Edbauer. Scheres wondered if the ribbon images might be sharpened by applying the averaging trick the authors used for identifying proteasomes.—Marina Chicurel
References
News Citations
- Tau Filaments from the Alzheimer’s Brain Revealed at Atomic Resolution
- New ALS Genes Implicate Protein Degradation, Endoplasmic Reticulum
Paper Citations
- Chang YJ, Jeng US, Chiang YL, Hwang IS, Chen YR. The Glycine-Alanine Dipeptide Repeat from C9orf72 Hexanucleotide Expansions Forms Toxic Amyloids Possessing Cell-to-Cell Transmission Properties. J Biol Chem. 2016 Mar 4;291(10):4903-11. Epub 2016 Jan 14 PubMed.
- Freibaum BD, Taylor JP. The Role of Dipeptide Repeats in C9ORF72-Related ALS-FTD. Front Mol Neurosci. 2017;10:35. Epub 2017 Feb 13 PubMed.
- Mackenzie IR, Frick P, Grässer FA, Gendron TF, Petrucelli L, Cashman NR, Edbauer D, Kremmer E, Prudlo J, Troost D, Neumann M. Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol. 2015 Dec;130(6):845-61. Epub 2015 Sep 15 PubMed.
- Mori K, Arzberger T, Grässer FA, Gijselinck I, May S, Rentzsch K, Weng SM, Schludi MH, van der Zee J, Cruts M, Van Broeckhoven C, Kremmer E, Kretzschmar HA, Haass C, Edbauer D. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 2013 Oct 17; PubMed. Correction.
- Oikonomou CM, Jensen GJ. Cellular Electron Cryotomography: Toward Structural Biology In Situ. Annu Rev Biochem. 2017 Jun 20;86:873-896. Epub 2017 Apr 19 PubMed.
- May S, Hornburg D, Schludi MH, Arzberger T, Rentzsch K, Schwenk BM, Grässer FA, Mori K, Kremmer E, Banzhaf-Strathmann J, Mann M, Meissner F, Edbauer D. C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol. 2014 Oct;128(4):485-503. Epub 2014 Aug 14 PubMed.
- Bäuerlein FJ, Saha I, Mishra A, Kalemanov M, Martínez-Sánchez A, Klein R, Dudanova I, Hipp MS, Hartl FU, Baumeister W, Fernández-Busnadiego R. In Situ Architecture and Cellular Interactions of PolyQ Inclusions. Cell. 2017 Sep 21;171(1):179-187.e10. Epub 2017 Sep 7 PubMed.
- Gorrie GH, Fecto F, Radzicki D, Weiss C, Shi Y, Dong H, Zhai H, Fu R, Liu E, Li S, Arrat H, Bigio EH, Disterhoft JF, Martina M, Mugnaini E, Siddique T, Deng HX. Dendritic spinopathy in transgenic mice expressing ALS/dementia-linked mutant UBQLN2. Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):14524-9. Epub 2014 Sep 22 PubMed.
- Wehmer M, Rudack T, Beck F, Aufderheide A, Pfeifer G, Plitzko JM, Förster F, Schulten K, Baumeister W, Sakata E. Structural insights into the functional cycle of the ATPase module of the 26S proteasome. Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):1305-1310. Epub 2017 Jan 23 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Guo Q, Lehmer C, Martínez-Sánchez A, Rudack T, Beck F, Hartmann H, Pérez-Berlanga M, Frottin F, Hipp MS, Hartl FU, Edbauer D, Baumeister W, Fernández-Busnadiego R. In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment. Cell. 2018 Feb 8;172(4):696-705.e12. Epub 2018 Feb 1 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Keio University School of Medicine
This is an excellent study, which shows direct impairment of proteasome by Poly-GA aggregates. Most of ALS/FTD-related molecules belong to two intracellular machineries involved in RNA regulation and protein quality control systems (Ito et al., 2017). Mutants in the latter (including p62, optineurin, TBK1, UBQLN2, VCP/p97, and CCNF) are assumed to lead to dysfunction of the proteasome and/or of autophagy, resulting in the accumulation of RNA-binding proteins, such as TDP-43, and hence RNA dysregulation. Therefore, this finding is strong evidence indicating a link between dipeptide repeat proteins and TDP-43 proteinopathy in C9 brain.
References:
Ito D, Hatano M, Suzuki N. RNA binding proteins and the pathological cascade in ALS/FTD neurodegeneration. Sci Transl Med. 2017 Nov 8;9(415) PubMed.
Make a Comment
To make a comment you must login or register.