Better than Crystal Clear? Cryo-EM Achieves Atomic Resolution
Quick Links
Monochromator? Check. Second-generation spherical aberration corrector? Check. Titan Krios G3 electron microscope? Check. And not to forget the Falcon 3 direct electron detector. Check.
- New hardware and software improve cryo-EM signal-to-noise ratio.
- Mouse apoferritin and human GABAA receptor resolved to atomic level.
- Other mysterious macromolecular structures may be next.
It may sound like science fiction, but this new combination of hardware allowed Holger Stark and colleagues at the Max-Planck Institute for Biophysical Chemistry, Göttingen, Germany, to break the atomic resolution barrier for cryo-EM. In the October 21 Nature, they report the structure of mouse apoferritin to a resolution of 1.25Å.
And they were not alone. Researchers led by Sjors Scheres and Radu Aricescu at the MRC Laboratory of Molecular Biology, Cambridge, U.K., pulled off the same feat, albeit with a slightly different device. They “made it so,” to paraphrase Starfleet Captain Jean-Luc Picard, with a next-generation Falcon 4 detector, a new cold field emission electron gun, and a new filter to correct for aberrant electrons scattered by the sample. Alas, no tachyon beams. In addition to a 1.22Å map of apoferritin, this new high-tech configuration resolved the structure of a human GABAA receptor down to 1.7Å. Their paper appears in the same issue of Nature.
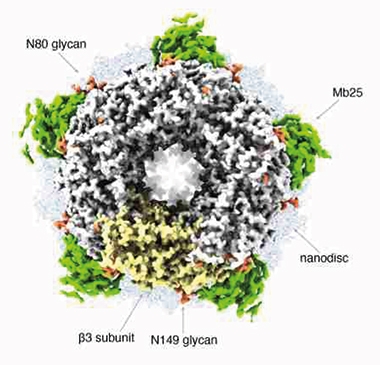
Perfect Pentamer. A 1.7Å cryo-EM structure, of human GABAA receptor lying on a lipid nanodisc and stabilized by a megabody protein scaffold (green), shows its pentameric ring surrounding its chloride channel. One of the subunits is colored yellow. Glycan modifications are orange. [Courtesy of Nakane et al., Nature 2020.]
“These developments will help researchers gain a better understanding, at unprecedented resolution, of how proteins work in health and disease, with the potential to aid the design of better therapeutics,” wrote Mark Herzik Jr., University of California, Davis, in a Nature News & Views. The advance has gotten the attention elsewhere, including in Science.
Many biological structures, especially those that embed themselves in membranes, are notoriously difficult to crystallize, leaving scientists with cryo-EM as the best option for solving detailed structures. Scheres told Alzforum that his lab is already trying the new microscope on amyloids that cause neurodegenerative diseases, such as Alzheimer’s. “Hopefully, this will lead to some eagerly awaited filament structures,” he wrote. Scheres’ group has previously solved structures of tau and α-synuclein filaments isolated from human brain, though at resolutions around 2.5 to 3.5Å (Jul 2017 news; Aug 2018 news; Mar 2020 conference news).
The unprecedented detail of the new structures rivals that of X-ray crystallography—even bests it when it comes to identifying hydrogen atoms and bonding, which predominate in most proteins. Hydrogen atoms are practically invisible in crystallography because their single electrons poorly diffract X-ray photons. Its nucleus, however, does a good job of scattering electrons. Hence, cryo-EM can identify hydrogen atoms at lower resolution than can crystallography.
Alas, a major problem has been holding back cryo-EM. It’s the signal-to-noise ratio. Because proteins are fragile, electrons fired their way must be relatively slow, and few and far between, so the protein does not disintegrate. As a result, the images obtained are fuzzy. To sharpen them, scientists take several hundred thousand or even millions of two-dimensional images of the same sample, and digitally average them to achieve a three-dimensional resolution of 2Å to 3Å. Obtaining a 1Å map of apoferritin with commonly used cryo-EM hardware would require several hundred billion images. That’s fine if you can store yottabytes of data and wait 200 years for the result.
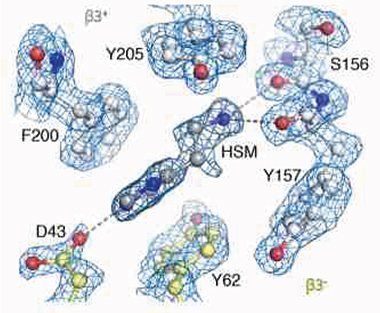
With Cryo-EM Glasses. 1.7Å map of the β3 GABAA receptor pocket reveals a histamine (HSM) bound to the carboxyl group of aspartic acid 43 (D43) on one end, and to glutamic acid 155 (not labeled) on the other. [Courtesy of Nakane et al., Nature 2020.]
First author Ka Man Yip and colleagues in Stark’s lab, and co-first authors Takanori Nakane, Abhay Kotecha, and Andrija Sente in Cambridge, improved the signal-to-noise ratio dramatically. Both groups used slightly different approaches to more narrowly focus the electrons hitting the sample, and to better capture and analyze those it scatters. Both approaches radically reduced the number of particle images needed for atomic-level detail of apoferritin. This protein has become a common benchmark for Cryo-EM studies because of its stability and 24-fold symmetry.
Yip resolved the structure to 1.25Å needing only 1 million particle images, while Nakane and colleagues got their 1.22Å map with even fewer, about 360,000 images—and it took only 36 hours. In a paper posted to bioRxiv on October 19, a third group, led by Kaiming Zhang and Wah Chiu at Stanford University, reported a 1.35Å map of apoferritin that took only 15 hours of data collection. Zhang and colleagues also used the new Falcon 4 detector and a data analysis scoring method they had previously developed to improve mapping resolution.
Beyond Apoferritin
Nakane and colleagues also tackled the β3 homopentameric GABAA receptor, which had eluded structural mapping below about 3Å (Jan 2019 news). GABA receptors play crucial roles in inhibitory neurotransmission, and having detailed structural information would help scientists better understand how they work. In fact, in the October 23 Neuron, scientists led by Richard Kramer at the University of California, Berkeley, reported that activity-dependent redistribution of extrasynaptic GABAA receptors to the synapse prevents runaway long-term potentiation—and preserves memory in mice (Davenport et al., 2021).
Due to its fivefold symmetry, Nakane and colleagues were able to obtain their 1.7Å map of β3 GABAA from about 370,000 particle images. The unprecedented level of detail shows how carboxyl groups from glutamic acid 155 and aspartic acid 43 coordinate to surround ligands that fill the agonist pocket (see image above). “Obtaining such a high resolution by single-particle cryo-EM had been deemed near impossible for a biological specimen such as this, one that exhibits a high level of flexibility in terms of its structural mobility,” wrote Herzik.
Scheres and colleagues believe the improved signal-to-noise ratio will expand both single-particle analysis and electron tomography, a type of cryo-EM for tissue, to now be able to take on more difficult samples, such as membrane proteins and large heterogeneous complexes. Herzik suspects that by melding the approaches adopted by the Göttingen and Cambridge groups, scientists might even break the 1Å barrier. “This once might have seemed a near impossible quest to embark upon,” he wrote.—Tom Fagan.
References
News Citations
- Tau Filaments from the Alzheimer’s Brain Revealed at Atomic Resolution
- Conformers Confirmed: Structure of Pick’s Tau Distinct from AD Tau
- Behold the First Human α-Synuclein CryoEM Fibril Structure
- GABA-A Receptor Structures Point to Drug Mechanisms
Paper Citations
- Davenport CM, Rajappa R, Katchan L, Taylor CR, Tsai MC, Smith CM, de Jong JW, Arnold DB, Lammel S, Kramer RH. Relocation of an Extrasynaptic GABAA Receptor to Inhibitory Synapses Freezes Excitatory Synaptic Strength and Preserves Memory. Neuron. 2020 Oct 14; PubMed.
External Citations
Further Reading
Primary Papers
- Yip KM, Fischer N, Paknia E, Chari A, Stark H. Atomic-resolution protein structure determination by cryo-EM. Nature. 2020 Oct 21; PubMed.
- Nakane T, Kotecha A, Sente A, McMullan G, Masiulis S, Brown PM, Grigoras IT, Malinauskaite L, Malinauskas T, Miehling J, Uchański T, Yu L, Karia D, Pechnikova EV, de Jong E, Keizer J, Bischoff M, McCormack J, Tiemeijer P, Hardwick SW, Chirgadze DY, Murshudov G, Aricescu AR, Scheres SH. Single-particle cryo-EM at atomic resolution. Nature. 2020 Oct 21; PubMed.
- Herzik MA Jr. Cryo-electron microscopy reaches atomic resolution. Nature. 2020 Oct 21; PubMed.
- Zhang K, Pintilie GD, Li S, Schmid MF, Chiu W. Resolving Individual-Atom of Protein Complex using Commonly Available 300-kV Cryo-electron Microscopes. bioRxiv. August 19, 2020.
- Davenport CM, Rajappa R, Katchan L, Taylor CR, Tsai MC, Smith CM, de Jong JW, Arnold DB, Lammel S, Kramer RH. Relocation of an Extrasynaptic GABAA Receptor to Inhibitory Synapses Freezes Excitatory Synaptic Strength and Preserves Memory. Neuron. 2020 Oct 14; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.