Breast Cancer Gene Implicated in Alzheimer’s
Quick Links
BRCA1, a DNA repair protein best known for its ties to breast cancer, appears to matter for Alzheimer’s disease as well. Scientists led by Lennart Mucke, Gladstone Institute of Neurological Disease, San Francisco, California, found that people with Alzheimer’s disease (AD) have less than half the normal amount of the protein in the hippocampus. The same is true in mice that model the disease. When the researchers reduced BRCA1 expression in wild-type mice, the animals accumulated DNA breaks, some of their neurons became dysfunctional, and they had trouble in learning and memory tests. The findings were published in the November 30 Nature Communications. Aβ appears to promote the destruction of BRCA1 in neurons, allowing DNA damage to go unrepaired. The results suggest that BRCA1 shields the neuronal genome and bolsters cognition, but its protection wanes in AD.
“This study, for the first time, implicates BRCA1 in cognitive function and maintenance of neuronal integrity,” Mucke told Alzforum. “We show that if you reduce BRCA1 levels after the brain has developed, you get changes reminiscent of Alzheimer’s disease.”
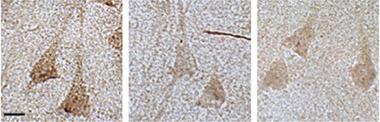
Dwindling BRCA1.
Hippocampal neurons from people with MCI (middle) and AD (right) contain less than half the BRCA1 (brown) of controls (left). [Courtesy of Suberbielle et al., Nat Commun.]
If it goes unchecked, damage to DNA can spell doom for cells, including neurons. Scientists have tied inadequate DNA repair to various neurodegenerative disorders, including AD, Huntington’s disease, and spinocerebellar ataxia. Previously, Mucke’s group found that normal activation of neurons caused cuts across both strands of the DNA (Mar 2013 news). Such double-strand breaks are one of the most deleterious types of DNA damage, because they can lead to large chromosome rearrangements, tumor formation, or apoptosis. In dividing cells, these breaks are repaired by homologous recombination, which requires an intact version of the identical DNA molecule to serve as a template. Non-homologous end joining can also fix these breaks, but it does so without a template strand and is more error-prone. It is the only way to fix double-strand breaks in non-dividing cells like neurons. Mucke found that double-strand breaks were quickly repaired in wild-type cells, but accumulated when Aβ levels rose, and stuck around longer in J20 transgenic mice, which overexpress human amyloid precursor protein (APP) with the Swedish and Indiana mutations. His group wondered if Aβ thwarts mechanisms of repair.
To find out, first author Elsa Suberbielle took a closer look in J20 mice at specific repair factors that fix double strand breaks, namely the proteins BRCA1, Rad51, NBS1, and MRE11. These proteins function in both non-homologous end joining and homologous recombination. Compared with controls, she found that transgenics had a dearth of one of these—BRCA1. Levels in the dentate gyrus parietal cortices were 30 and 55 percent, respectively, of those in wild-type mice. Likewise, postmortem brain samples from people who had had AD or mild cognitive impairment had just 25 to 35 percent of the usual BRCA1 protein in the hippocampus (see image above). BRCA1 gloms onto double-strand breaks in DNA and recruits other proteins that glue the ends back together, mediating the DNA repair process. The function of BRCA1 in non-homologous end joining has been studied mostly in dividing cells. How it works in neurons is still unclear.
To determine how a drop in BRCA1 affects neurons, Suberbielle and colleagues used small-hairpin RNAs (shRNA) to knock down the protein in neurons of the dentate gyrus in mice. BRCA1 reduction led to the buildup of double-strand breaks in DNA. If these neurons expressed low amounts of human APP, they amassed even more damage. BRCA1 reduction also shrank neuronal cell bodies and dendritic arbors. Hippocampal slices from mice injected with shRNA mustered only weak long-term potentiation, a form of synaptic plasticity, which suggested BRCA1 helps govern learning and memory. This bore out in tests of mouse behavior. In the Morris water maze, mice injected with the BRCA1 small-hairpin RNA had trouble learning and remembering the location of a hidden platform. J9 mice that produce about half the amount of human APP of J20 mice navigated even more poorly. This implies that Aβ exacerbates the problems caused by the loss of BRCA1.
What links Aβ to BRCA1? Evidence suggests Aβ stimulates NMDA receptors that lie outside the synapse. Mucke had previously found that activating extrasynaptic NMDA receptors led to more double-strand breaks in DNA. In the current work, he found that stimulating these receptors in cultured primary hippocampal neurons cut BRCA1 levels in half, as did bathing the cells with Aβ oligomers. Blocking protein degradation by the proteasome prevented the loss of BRCA1, suggesting that protein clearance was to blame.
The researchers do not yet know how double-strand breaks lead to altered form and function of neurons, but they speculate that they influence gene expression. In cultured hippocampal neurons, they found that reducing BRCA1 caused histone modifications previously associated with the repression of genes.
“This study adds to previous evidence that DNA damage leads to changes in gene expression that may contribute to cognitive decline,” said Li-Huei Tsai, Massachusetts Institute of Technology, Cambridge, Massachusetts (see Jun 2004 news on Lu et al., 2004). That neurons did not die suggests that any gene expression change caused by DNA damage represents an early step in the disease process, Tsai added. She pointed out that the authors looked at only a few repair factors; more could be affected in Alzheimer’s.
Do BRCA1 mutations that increase cancer risk lead to Alzheimer’s as well? Mucke said no evidence exists to suggest carriers are more susceptible to AD. He pointed out that the brain might adapt to mutations that alter the protein from birth. To test this, his group will analyze databases of mutation carriers whose cognition has been tested. The researchers will also examine how other components of DNA repair are affected in AD and whether BRCA1 levels influence other neurodegenerative diseases. In addition, they want to understand whether elevating BRCA1 levels prevents some of the problems brought on by Aβ.
Mounting evidence suggests that neuronal DNA damage occurs in Alzheimer’s, said Mark Mattson, National Institute on Aging, Baltimore, Maryland. He noted that most studies have focused on base-excision repair, which targets single DNA bases that have undergone oxidative damage. Mattson and colleagues reported that this repair process falters in patients with AD and MCI and that suppressing base-excision repair leads to neuronal dysfunction and death, as well as impaired memory and synaptic plasticity (Weissman et al., 2007; Sykora et al., 2015). “This new paper nicely shows that BRCA1 protects against DNA damage in neurons,” he said. Since it is unclear how BRCA1 works in these cells, he speculated that the protein could function in other ways that do not involve double-strand breaks, a point conceded by the authors.—Gwyneth Dickey Zakaib
References
News Citations
- Aβ, Neural Activity Linked to DNA Damage
- After 40, DNA Damage Accrues in Genes, Hampering Expression
Research Models Citations
Paper Citations
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004 Jun 24;429(6994):883-91. PubMed.
- Weissman L, Jo DG, Sørensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35(16):5545-55. PubMed.
- Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D, Tian J, Baptiste BA, Cong WN, Brenerman BM, Fang E, Becker KG, Hamilton RJ, Chigurupati S, Zhang Y, Egan JM, Croteau DL, Wilson DM 3rd, Mattson MP, Bohr VA. DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res. 2015 Jan;43(2):943-59. Epub 2014 Dec 30 PubMed.
Further Reading
Papers
- Madabhushi R, Pan L, Tsai LH. DNA damage and its links to neurodegeneration. Neuron. 2014 Jul 16;83(2):266-82. PubMed.
- Silva AR, Santos AC, Farfel JM, Grinberg LT, Ferretti RE, Campos AH, Cunha IW, Begnami MD, Rocha RM, Carraro DM, de Bragança Pereira CA, Jacob-Filho W, Brentani H. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer's disease. PLoS One. 2014;9(6):e99897. Epub 2014 Jun 17 PubMed.
Primary Papers
- Suberbielle E, Djukic B, Evans M, Kim DH, Taneja P, Wang X, Finucane M, Knox J, Ho K, Devidze N, Masliah E, Mucke L. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun. 2015 Nov 30;6:8897. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.