CD22 Suppresses Microglial Phagocytosis—A New Therapeutic Target?
Quick Links
Microglia tend to get off-kilter in Alzheimer’s and other neurodegenerative diseases. Now, researchers in Tony Wyss-Coray’s lab at Stanford University believe they have discovered a means of restoring their homeostasis. On April 3 in Nature online, they reported that blocking CD22, a cell surface receptor associated with B cells, reprograms microglia to look more like those in young, healthy mice, the scientists contend. The blockade unleashed phagocytosis of aggregated Aβ, α-synuclein, and other cellular debris in old mice, and improved their memories.
- CD22 popped up in screening for suppressors of microglial phagocytosis.
- The receptor is upregulated in aging mice and in Alzheimer’s.
- Blocking CD22 improves phagocytosis and memory in old mice.
“I think the most exciting aspect is that microglia almost act like B cells,” said Wyss-Coray. Decades of research have outlined how signaling through CD22, as in cluster of differentiation, suppresses B cells after they recognize an antigen. This is a control mechanism to prevent overactivation of an immune response. In aging microglia, the Stanford scientists now propose, CD22 may similarly be suppressing normal functions, such as mopping up cellular debris and toxic proteins.
First author John Pluvinage and colleagues identified CD22 in a CRISPR-based search for transcripts that suppress or enhance phagocytosis. The researchers used 30,000 guide RNAS to target about 3,000 genes, including 950 transmembrane proteins and about 2,000 known drug targets. They first screened BV2 microglia-like cells by CRISPR, then compared that data to RNA-Seq transcriptional profiles of microglia from young and aged mice. They were searching for phagocytosis suppressors or enhancers that change with age.

Unlocking Phagocytosis. Mice co-injected with an anti-CD22 antibody (right) cleared injected Aβ oligomers (red) within two days, while a control IgG had no effect. Microglial numbers, as judged by Iba1 staining (bottom), did not change. [Courtesy of Nature, Tony-Wyss Coray.]
Only CD22 fit the bill. Knocking it out with CRISPR boosted phagocytosis in BV2 cells, while microglia from older mice had fivefold more CD22 than did cells from young mice, as reported previously (Hickman et al., 2013; Grabert et al., 2016). CD22 also appears to be upregulated in AD (Friedman et al., 2018).
CD22 belongs to the family of sialic acid-binding immunoglobulin-like lectins, or Siglecs, which also includes CD33. Variants in CD33 suppress Aβ phagocytosis and increase risk for Alzheimer’s disease (Griciuc et al., 2013; Aug 2013 news).
To test if CD22 acts similarly, Pluvinage injected Aβ oligomers bilaterally into the brains of 14- to 16-month-old mice, adding an antibody against CD22 to one hemisphere and a control IgG to the other. The anti-CD22 treatment spurred clearance of Aβ within two days (see image below). In separate experiments, anti-CD22 similarly cleared injected myelin and α-synuclein fibrils. Taking a different approach, the authors found they could inhibit phagocytosis using lipid-bound synthetic glycopolymers that mimic the sialic acid ligands that activate CD22 (see image below). These failed to block phagocytosis in CD22 knockout cells.
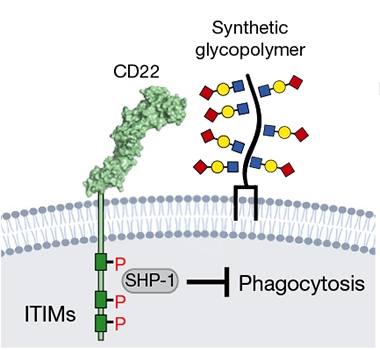
CD22 stops phagocytosis. Synthetic glycopolymers of sialic acid bind CD22, activate SHP-1 and put the brakes on phagocytosis. [Courtesy of Tony Wyss-Coray, Nature.]
How would CD22 blockade promote microglial phagocytosis? CD22 recruits tyrosine phosphatase SHP-1/PTPN6 to inhibit immune receptor signaling and Wyss-Coray believes CD22 antibodies antagonize inhibitory signaling in phagocytic receptors, thereby restoring phagocytosis. “What is really surprising is that the whole transcriptional profile of these cells changes, not just phagocytosis,” said Wyss-Coray. After one month of continuous CD22 blockade, microglia from 18-month-old wild-type mice assumed a transcriptional profile resembling that of microglia from 3-month-old mice. They had turned down expression of genes upregulated during aging, and increased expression of genes suppressed by age. In each case, genes associated with microglial homeostasis, such as P2ry13, Sall1, Il10ra, and Mef2a, were more active, and disease-associated genes, including Tspo, Lgals3, Tnfsf13b and Ccl3, shut down (Jun 2017 news). In the old mice, this shift appeared to improve synaptic plasticity, as indicated by more c-fos in the dentate gyrus. They also spent more time exploring a novel object in a Y maze, and froze longer in a contextual fear experiment, both indications of better memory.
Wyss-Coray is exploring how to exploit this receptor to treat AD and potentially other neurodegenerative diseases of aging.
Alector, a biotech company in South San Francisco, has begun a Phase I clinical trial of an anti-CD33/Siglec3 antibody in healthy controls and AD patients.—Tom Fagan.
References
News Citations
Paper Citations
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013 Dec;16(12):1896-905. Epub 2013 Oct 27 PubMed.
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016 Mar;19(3):504-16. Epub 2016 Jan 18 PubMed.
- Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, Cao Y, Lee SH, Haddick PC, Ngu H, Modrusan Z, Larson JL, Kaminker JS, van der Brug MP, Hansen DV. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer's Disease Not Evident in Mouse Models. Cell Rep. 2018 Jan 16;22(3):832-847. PubMed.
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013 May 22;78(4):631-43. PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Pluvinage JV, Haney MS, Smith BA, Sun J, Iram T, Bonanno L, Li L, Lee DP, Morgens DW, Yang AC, Shuken SR, Gate D, Scott M, Khatri P, Luo J, Bertozzi CR, Bassik MC, Wyss-Coray T. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019 Apr;568(7751):187-192. Epub 2019 Apr 3 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
Washington University in St. Louis
Pluvinage et al. elucidate previously unappreciated roles for CD22 in the aging brain. Comparing young and old mice, the group of Tony Wyss-Coray observed that CD22 is increased in microglia during aging. This observation was rather unexpected as CD22 is an inhibitory molecule mainly known to “dampen” the activation of B-lymphocytes.
Using advanced techniques of genetic engineering, the authors have shown that the age-dependent increase of CD22 in microglia correlates with a reduced phagocytic activity of these cells, resulting in accumulation of cellular debris and protein aggregates, which normally accumulate in the senile brain. It is remarkable that administration of a CD22 blocker in the brains of aged mice boosts the phagocytic function of microglia. Microglia could more efficiently remove both cellular debris and Aβ aggregates injected into the mouse brain. Importantly, the cognitive skills of old mice receiving the CD22 blocker, but not the placebo, were significantly improved.
These findings may soon pave the way for therapeutic applications in humans, especially for the treatment of age-related diseases such as Alzheimer’s.
Indiana University School of Medicine
New work from the Wyss-Coray lab reports that CD22 plays a role in regulating the phagocytic capacity of microglia. This is a compelling paper that adds depth to our understanding of the intricate mechanisms that act to regulate microglial actions. CD22 is principally expressed on B cells, thus its appearance on aged microglia is unexpected, although several recent RNA-Seq studies revealed its presence in the brain in other disease settings. The selective expression of CD22 as a function of aging provides a new and important example of how the aging brain employs sophisticated mechanisms to calibrate its capacity to perform tissue maintenance and constrain microglial responses to a progressively more proinflammatory brain microenvironment. CD22 has been described to act broadly to inhibit B-cell receptor signaling and although the present study is focused on phagocytosis, it is likely to have broader roles in suppressing microglial activation given the mechanisms through which it acts intracellularly.
It is interesting that a screen for modifiers of phagocytosis turned up CD22 in microglia. It should be noted their assay involved phagocytosis of fluorescent beads, which are taken up through fluid phase phagocytic mechanisms largely related to process extension and retraction. An analogous CRISPR screen of phagocytic regulators employed a rather different strategy and was designed to screen cargo-driven phagocytic mechanisms in myeloid cells (Haney et al., 2018). Interestingly, this screen also picked up genes associated with sialic acid moieties that were linked to suppression of phagocytosis.
One of the most intriguing outcomes is the capacity of CD22-null mice or those receiving anti-CD22 antibodies to perform better in several behavioral assays. These data serve to reinforce the idea that the modulation of the innate immune system in the brain plays critical roles in cognition in aging and dementia and may be amenable to therapeutic intervention.
Overall, this is a persuasive and impressive body of work, elegantly done.
Make a Comment
To make a comment you must login or register.