Chimeric Protein Dephosphorylates Tau, Improves Memory
Quick Links
Scientists are exploring many ways to tackle tau pathology. In the October 17 Cell Chemical Biology, researchers led by Jian-Zhi Wang at the Huazhong University of Science and Technology, Wuhan, China, advocated for a new one. They generated a chimeric protein, D20, that selectively recruits protein phosphatase 1 (PP1) to tau. PP1 then snips off phospho groups at several AD-related sites. In two tauopathy mouse models, peripheral administration of D20 lowered p-tau and total tau in the brain. Chronic dosing with D20 restored synaptic density and memory to normal.
- A chimeric protein selectively recruits protein phosphatase 1 to tau.
- In tauopathy mice, the chimera entered brain and lowered p-tau and t-tau to wild-type levels.
- This restored synaptic density and memory to normal.
Similar chimeras could be constructed to selectively target other pathologic proteins, the authors noted. “This study introduces a promising strategy for drug discovery,” they wrote.
The authors had previously experimented with using dephosphorylation targeting chimeras (DEPTACs) to bring phosphatases to tau. This strategy avoids the off-target effects of indiscriminately boosting phosphatase activity, or globally suppressing kinases, since these enzymes have many substrates. In initial studies, Wang and colleagues generated a DEPTAC that recruited protein phosphatase 2A to tau, lowering p-tau and t-tau in mice (Zheng et al., 2021). Scientists led by Craig Crews at Yale University, New Haven, Connecticut, have tried a similar approach (Hu et al., 2023).

Chimeric Coupler. The constructed molecule D20 links a tau-binding motif (green) to a protein phosphatase 1 binder (tan). It brings tau and PP1 together, allowing the enzyme to snip phospho groups off tau. [Courtesy of Xiao et al., Cell Chemical Biology.]
However, that first DEPTAC was effective only at a high dose of 200 μM. Joint first authors Yue Xiao and Linyu Wei set out to optimize it, screening a library of different DEPTACs. These molecules have four parts: a tau-binding motif, a linker region, a phosphatase-recruiting site, and finally a membrane-penetrating peptide at the C-terminal end. While experimenting, Xiao and Wei found that recruiting PP1 performed better than did PP2A at lowering p-tau. The scientists also improved the linker and membrane-penetrating sequence, and chose a tau-binding motif that was selective for fibrils over monomers. The new construct, D20, consisted of 23 amino acids plus a polyethylene glycol linker (image below).
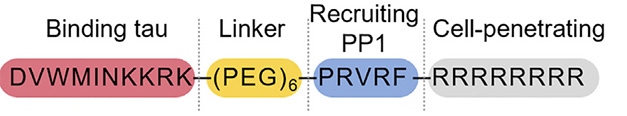
DEPTAC. Based on PROTACs, or protein-targeting chimeras, the dephosphorylation-targeting chimera D20 delivers PP1 to tau. Its C-terminal tail (gray) helps the construct enter cells. [Courtesy of Xiao et al., Cell Chemical Biology.]
In rat primary neurons, D20 worked, recruiting PP1 to tau. At a dose of 25 μM, it nearly eliminated phosphorylation at several sites within six hours. These included serines 199, 396, and 404, and threonines 181, 205 and 262. In P301L mice, a single dose of 1 mg/kg D20 into the tail vein halved p-tau and t-tau after 24 hours.

Synaptic Savior. 3xTg tauopathy mice (middle) lose dendrites and spines by 10 months (close-up at right), but chronic dosing with the D20 chimera for 20 days (bottom) restores these to nearly wild-type levels (top). [Courtesy of Xiao et al., Cell Chemical Biology.]
To test chronic dosing, the authors chose 3xTg mice, which carry the P301L tau variant as well as two amyloid plaque-promoting mutations. Every other day for 20 days, the authors injected D20 into the tail veins of 10-month-old 3xTg mice, which have severe tau pathology. The treatment suppressed p-tau by one-half to three-fourths, while lowering t-tau to the levels in wild-type mice. Dendritic arborization and spine density returned to wild-type levels as well (image at right). Neurons stopped dying, and neuronal calcium responses and neurogenesis bounced back to normal.
In keeping with this, the mice performed like wild-types in several behavioral tests. They remembered foot shocks, objects they had seen before, and the location of a hidden platform in the Morris water maze. Importantly, the mice did not show signs of liver, renal, or cardiac toxicity.
At the same time, D20 worked poorly in a third tauopathy mouse model, which expresses the neurotoxic N368 tau fragment via an adenovirus. Possibly, this tau fragment interacts differently with D20, the authors suggested. “This highlights the necessity for customized therapeutic strategies when addressing the complexity of tauopathies … a universal approach may not be effective,” the authors noted.—Madolyn Bowman Rogers
References
Research Models Citations
Paper Citations
- Zheng J, Tian N, Liu F, Zhang Y, Su J, Gao Y, Deng M, Wei L, Ye J, Li H, Wang JZ. A novel dephosphorylation targeting chimera selectively promoting tau removal in tauopathies. Signal Transduct Target Ther. 2021 Jul 14;6(1):269. PubMed.
- Hu Z, Chen PH, Li W, Douglas T, Hines J, Liu Y, Crews CM. Targeted Dephosphorylation of Tau by Phosphorylation Targeting Chimeras (PhosTACs) as a Therapeutic Modality. J Am Chem Soc. 2023 Feb 8; PubMed.
Further Reading
News
- Put a RING on It: Targeting Intracellular Tau Aggregates for Destruction
- p-Tau217 Immunotherapy Alleviates Tangle Pathology in Mice
- Therapeutic Contenders Target Hard-to-Reach Pockets of Tau
- Moving Forward: RNA-Targeted Attempts at Taking Down Tau, APP
- New Arrows Aimed at Tau: Single-Domain Antibody, Peptibody, Vaccine
- Paper Alert: Tau Antisense Oligonucleotide BIIB080 Hits Its Target
- Two New Stabs at Vaccinating People Against Pathologic Tau
Primary Papers
- Xiao Y, Wei L, Su J, Lei H, Sun F, Li M, Li S, Wang X, Zheng J, Wang JZ. A tau dephosphorylation-targeting chimeraselectively recruits protein phosphatase-1 to ameliorate Alzheimer's disease and tauopathies. Cell Chem Biol. 2024 Oct 17;31(10):1787-1799.e6. Epub 2024 Sep 30 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Laboratory for Alzheimer Disease
The propagation of neurofibrillary tangles (NFTs) from the entorhinal cortex to the limbic system and neocortex is known to correlate with cognitive decline due to accompanying neuronal loss. Tau, which constitutes NFTs, is excessively phosphorylated, and attempts have been made to inhibit this by blocking the kinase GSK-3β or by activating phosphatases. However, these approaches have not succeeded as treatments for dementia. The issue lies in the fact that kinases that phosphorylate tau or phosphatases that dephosphorylate it have many substrates other than tau; inhibiting or activating these can affect many cellular functions and potentially cause adverse side effects.
In this paper, Xiao et al. successfully used the D20 peptide to recruit protein phosphatase 1 (PP1) and specifically dephosphorylate tau molecules, ingeniously solving the problem of side effects by conferring tau specificity to PP1. The next question might be whether excessive phosphorylation is the cause or result of NFT formation.
At least our results indicate that phosphorylation at microtubule-binding sites S262 and S356 causes tau to dissociate from microtubules and form droplets through liquid-liquid phase separation (Soeda et al., 2024). On the other hand, when these sites are dephosphorylated and tau binds to microtubules, aggregated tau becomes aggresome-like and seems to be transported to protein degradation systems; thus, excessive phosphorylation of tau may indeed be a cause of NFTs and associated dementia. To date, therapies targeting tau have not been successful in clinical trials, in contrast to those targeting Aβ. Starting with Xiao et al.'s findings, we hope for the development of new therapeutic methods targeting tau.
References:
Soeda Y, Yoshimura H, Bannai H, Koike R, Takashima A. Intracellular Tau Fragment Droplets Serve as Seeds for Tau Fibrils. 2023 Sep 11 10.1101/2023.09.10.557018 (version 1) bioRxiv.
Make a Comment
To make a comment you must login or register.