At the Core of Microglial Signaling: γ-Secretase
Quick Links
Nestled within the plasma membrane, γ-secretase trims the ends off membrane proteins. Among its 150 known substrates, none is more famous than APP. The protease chews APPs transmembrane domain to spit out Aβ peptides that aggregate into amyloid plaques. When inhibitors of this APP processing worsened cognition in Alzheimer's disease trials, scientists thought the reason was that the secretase also cleaves dozens of other proteins.
- A new method identified dozens of γ-secretase substrates—in microglia.
- Many control these cells' responses.
- Without γ-secretase, the cells all but ignore amyloid plaques.
Now, scientists led by Bart De Strooper at KU Leuven in Belgium have identified dozens of γ-secretase substrates in microglia alone. In the November 16 Molecular Cell, they reported that without the secretase, microglia mostly ignore plaques, rather than flocking to them to devour and contain them. The results offer a new explanation for why inhibitors flopped in trials. De Strooper, who also directs the UK Dementia Research Institute at University College London, thinks γ-secretase modulators will have more success.
“This intriguing and refreshingly solid study maps the interaction network of γ-secretase in microglia and provides valuable information on the role of γ-secretase in microglia biology,” wrote Thomas Südhof of Stanford University, California. Marco Colonna and Yun Chen of the Washington University School of Medicine in Saint Louis also praised the work. “The researchers performed a series of intriguing experiments focusing on the potential substrate map of one of the most promiscuous enzymes, γ-secretase, and its role in microglial activation,” they wrote (comment below).
Although γ-secretase cleaves approximately 150 membrane proteins, most studies have focused on one substrate at a time. While this provides insight into the fate of that one protein, it does not capture the secretase's physiological repertoire. To do that, the entire γ-secretase proteome, i.e., every protein it trims at any given time, needs to go under the microscope.
For γ-secretase, identifying its substrates has been challenging. Due to its aspartyl protease activity, no chemical chicanery has been found that covalently labels substrates for identification, as can be done for cysteine proteases, for example. And unlike membrane-bound serine proteases, γ-secretase does not cleave at a consensus sequence that in silico sequence alignment tools can easily spot.
Instead, co-first authors Pengfei Hou and Magdalena Zielonka turned to γ-secretase inhibition to identify substrates. Membrane protein C-terminal fragments that accumulate in inhibitor-treated cells would be likely substrates, they reasoned. They developed an unbiased screen dubbed γ-secretase substrate identification (G-SECSI; image below). They treated H9 embryonic stem cell-derived microglia-like cells with semagacestat. Then then separated membrane proteins by size using gel-eluted liquid fraction entrapment electrophoresis, aka GELFrEE. It allows further analysis without needing to extract proteins from bits of gel. Each fraction underwent mass spectrometry to identify full-length proteins and accumulated C-terminal fragments.

Substrates, Anyone? To catalog the microglial γ-secretase proteome, GELFrEE fractions were analyzed by mass spectrometry. [Courtesy of Hou et al., Molecular Cell, 2023.]
Among approximately 4,300 peptides from 360 membrane proteins, 85 fit the bill (image below). Of these, 59 had not been reported as γ-secretase substrates before. Many are receptor protein kinases or proteins that bind cytokines, suggesting roles in cell-cell signaling. Six receptor tyrosine kinases, including the disease-associated microglia (DAM) gene AXL, four interleukin receptors, three tumor necrosis factor receptors, and two colony-stimulating factor receptors vital to microglial survival and proliferation made the list.
Intriguingly, in a cerebrospinal fluid proteomic analysis, Betty Tijms of the Amsterdam University Medical Center and Pieter Jelle Visser at Maastricht University, also in the Netherlands, found that 66 of these 85 "Leuven" proteins contributed to a panel of 1,492 proteins that define a subtype of AD characterized by innate immune activation (Aug 2019 conference news; Tijms et al., 2023; story to come). People with this AD subtype had the highest levels of BACE1, Aβ40, and other markers of amyloid metabolism in their CSF. “The Hou et al. study suggests that possibly a microglial γ-secretase response may explain such high levels,” wrote Visser and Tijms (comment below). “This would be an interesting question to further investigate.”
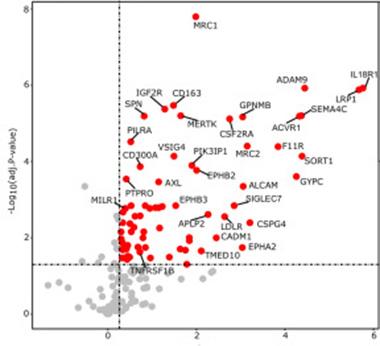
γ-Secretase Proteome. This volcano plot shows 85 proposed substrates in microglia, and the buildup of their C-terminal fragments upon γ-secretase inhibition. [Courtesy of Hou et al., Molecular Cell, 2023.]
Notably, 53 of the 85 γ-secretase substrates help describe microglial states. Of these, 26 control homeostasis, and 16 are encoded by DAM, interferon-response (IRM), or human leukocyte antigen (HLA) genes. DAMs, IRMs, and HLA microglia represent three distinct activation states.
Might γ-secretase influence microglial transitions into these different states? To find out, Hou and Zielonka analyzed single-cell transcriptomes of 8,600 H9 cultured microglial cells before and after semagacestat treatment. They saw two cell clusters: homeostatic and DAM-like. Among the untreated, 36 percent of the cells were homeostatic, 64 percent were DAMs, the latter possibly a reflection of microglia being in culture. Microglia are notoriously reactive and known to change state once removed from the brain (Jul 2016 conference news). After semagacestat treatment, 42 percent of the microglia were homeostatic, 58 percent DAM. About 350 genes accounted for this slight shift, with homeostatic gene expression increasing and DAM gene expression decreasing.
In mouse brain, the opposite happened. The scientists discovered this once they created conditional γ-secretase knockouts, in which adding tamoxifen snips out the APH1A γ-secretase subunit only in microglia. The protease needs four subunits to assemble: APH1, nicastrin, PEN-2, and presenilin, which harbors the active site. Hou and Zielonka fed tamoxifen to 1-month-old mice for five days, then analyzed their microglial transcriptome at 3 months using single-cell RNA-Seq. Mice deficient in γ-secretase had slightly more DAMs and slightly fewer homeostatic cells.
Why did the shift go in opposite directions in vivo and in culture microglia? De Strooper believes that the absence of γ-secretase from microglia doesn't simply shift them from one state to another. Rather, it lets microglia drift away from the “normal” condition—homeostasis in the case of a healthy mouse brain, inflammation in the case of cultures. “It really highlights that one should look at the integrated effects of all γ-secretase substrates on transcription,” De Strooper told Alzforum.
To learn if γ-secretase deficiency affects microglial responses to amyloid plaques, the scientists crossed the conditional γ-secretase knockouts with APP NL-G-F knock-in mice. They fed 1-month-old offspring tamoxifen to knock out microglial γ-secretase, then analyzed single-cell microglial transcriptomes at 3 or 7 months, when the mice had mild or severe plaque pathology, respectively. As in cell culture experiments, γ-secretase-deficiency in the mice left microglia stuck in homeostasis. Fewer DAMs emerged at 3 months, fewer still at 7, a finding reflected in upregulation of homeostatic and downregulation of DAM genes.
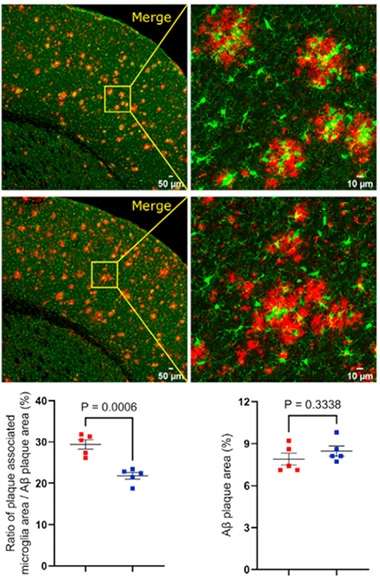
γ-Secretase, the Mobilizer. Microglia (green) swarmed amyloid plaques (red) in APP NL-G-F mice (top), less so when their γ-secretase was gone (middle). Bar plots show plaque-associated microglia (left) and plaques (right) in controls (red) and knockouts (blue). [Courtesy of Hou et al., Molecular Cell, 2023.]
How about plaques? Though the microglial y-secretase knockouts retained as many microglia as control mice, γ-sec ko microglia idled in the parenchyma rather than rushing to encircle amyloid plaques (image at right). Without γ-secretase, microglia did not respond normally. That said, equal amounts of amyloid aggregated in both mouse models.
All told, the authors conclude that γ-secretase integrates extracellular signals by cleaving an array of transmembrane proteins, each prompting its own signaling cascade to effect microglial reactions.
As such, the data help explain the failure of γ-secretase inhibitors. “The indiscriminate inhibition of γ-secretase to lower Aβ production in AD … is predicted to cause microglial dysfunction,” the authors wrote. “This may ultimately have contributed to the failure of the semagacestat Phase 3 clinical trial in 2013.” De Strooper noted that on-target side effects of secretase inhibitors are almost impossible to predict, or control.
To Jean-François Blain, Arkuda Therapeutics, Watertown, Massachusetts, this does not diminish γ-secretase as a drug target, though inhibition may not be the way. “I believe now is the time to think about γ-secretase modulators (GSMs) again, and how they could be used in prevention trials” (comment below). Roche is taking their GSM into Phase 2 (Nov 2023 conference news).
De Strooper agreed. He believes GSMs, which tweak secretase-APP binding to drive processing toward shorter Aβ peptides, should have little to no effect on the enzyme’s intracellular signaling and therefore, few side effects. De Strooper is testing this now by measuring how a handful of GSMs affect the secretase proteome and cell states of microglia.—Chelsea Weidman Burke
References
Therapeutics Citations
News Citations
- Proteomics Uncovers Potential Markers, Subtypes of Alzheimer’s
- When a Microglia Is No Longer a Microglia
- Second-Generation γ-Secretase Modulator Heads to Phase 2
Research Models Citations
Paper Citations
- Tijms BM, Vromen EM, Mjaavatten O, Holstege H, Reus LM, vanderLee SJ, Wesenhagen K, Lorenzini L, Vermunt L, Venkatraghavan V, Tesi N, Tomassen J, denBraber A, Goossens J, Vanmechelen E, Barkhof F, Pijnenburg YA, vanderFlier WM, Teunissen CE, Berven F, Visser PJ. Large-scale cerebrospinal fluid proteomic analysis in Alzheimer's disease patients reveals five molecular subtypes with distinct genetic risk profiles. 2023 May 11 10.1101/2023.05.10.23289793 (version 1) medRxiv.
Further Reading
No Available Further Reading
Primary Papers
- Hou P, Zielonka M, Serneels L, Martinez-Muriana A, Fattorelli N, Wolfs L, Poovathingal S, T'Syen D, Balusu S, Theys T, Fiers M, Mancuso R, Howden AJ, De Strooper B. The γ-secretase substrate proteome and its role in cell signaling regulation. Mol Cell. 2023 Nov 16;83(22):4106-4122.e10. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
Washington University School of Medicine
This paper represents impressive work. Hou et al performed a series of intriguing experiments, focusing on the potential substrate map of one of the most promiscuous enzymes–γ-secretase–and on its role in microglial activation.
First, they conducted mass spectra analysis on tris-acetate-gel-separated membrane protein extracts and screened for potential γ-secretase substrates by identifying undigested peptide sequences with semagacestat inhibition. The results revealed many new potential substrates of γ-secretase, with most hits appearing to be immunoreceptors characterized in various microglial states, including disease-associated microglia and interferon response microglia.
Second, they performed single-cell RNA sequencing on semagacestat-treated versus untreated H9MG microglial cells with lipopolysaccharide treatment, and found a consistent downregulation of genes associated with activation (some of which are shared with DAM) in the semagacestat-treated conditions.
Third, they analyzed microglial signatures in vivo in microglia γ-secretase conditional knockout mice (GSiΔMG) and in crosses with APPNL-G-F mice, and confirmed the suppression of microglial transcriptomic changes associated with activated states, including DAM, where most Aβ-induced microglial activation was trapped in pre-DAM and homeostatic states.
The study employed an elegant screening assay design. Previous literature (Tagami et al., 2017) reported that semagacestat suppresses release of γ-secretase products from the membrane, fostering the discovery of new but rare substrates in this study. However, given that many protein hits might also be involved in endosomal/lysosomal trafficking and are digested in lysosomes, it would be necessary to further verify each potential γ-secretase substrate with biochemical assays and other γ-secretase inhibitors.
Regardless, the global changes in microglial activity are convincing and may impact our understanding of the current γ-secretase inhibitor trials for cancer.
From our perspective, the results of fewer DAM signatures in APPNL-G-F-GSiΔMG mice, as well as the halt of microglia in the pre-DAM cluster, are fascinating. The authors concluded that this is a consequential phenotype of γ-secretase depletion, causing less microglial activity in response to amyloid pathology. It is possible that γ-secretase depletion may have a differential impact of activating versus inhibiting microglial receptors. Perhaps it would be worthwhile to further analyze the ratio between inhibitory receptors versus activation receptors affected by γ-secretase from figures 1 and 2, which may provide more insight into γ-secretase’s role in regulating microglial/myeloid cell response.
There might be an alternative hypothesis: The knockout of γ-secretase may preserve enough receptors and signaling to maintain individual microglial functions, leading to fewer transcriptomic changes as a secondary effect. Additionally, it is possible that only a portion of the microglial genes will be turned on due to the change in the uncleaved receptor repertoire, genes that had not been specifically characterized as being in the pre-DAM cluster in previous studies. Future proteomics analysis of sorted microglia populations will be important to approve or disapprove those hypotheses.
Notably, a recent study by Essayan-Perez and Südhof showed that γ-secretase inhibition led to cholesterol changes only in neurons but not in microglia. It will be interesting to compare these data with the data by Hou et al., and see whether microglia have better compliance than neurons to inhibition of γ-secretase.
References:
Tagami S, Yanagida K, Kodama TS, Takami M, Mizuta N, Oyama H, Nishitomi K, Chiu YW, Okamoto T, Ikeuchi T, Sakaguchi G, Kudo T, Matsuura Y, Fukumori A, Takeda M, Ihara Y, Okochi M. Semagacestat Is a Pseudo-Inhibitor of γ-Secretase. Cell Rep. 2017 Oct 3;21(1):259-273. PubMed.
Essayan-Perez S, Südhof TC. Neuronal γ-secretase regulates lipid metabolism, linking cholesterol to synaptic dysfunction in Alzheimer's disease. Neuron. 2023 Oct 18;111(20):3176-3194.e7. Epub 2023 Aug 4 PubMed.
Amsterdam UMC, loc. VUmc
Maastricht University; VU University Medical Centre
We checked to what extent the 85 γ-secretase substrates described in figure 2b of this paper were associated with distinct Alzheimer’s disease molecular subtypes we identified (Tijms et al., 2023). These AD subtypes were defined based on untargeted CSF proteomics in individuals with AD; they included a subtype with aberrant plasticity, a subtype with immune activation, a subtype with RNA dysfunction, a subtype with choroid plexus dysfunction, and a subtype with blood-brain barrier dysfunction.
Of the 85 γ-secretase substrates identified by Hou et al., we detected 66 in our CSF proteomic dataset. This means that those proteins might have use for measuring effects of γ-secretase inhibition in patients.
The majority of the 66 γ-secretase substrates had increased CSF levels in the AD subtype with innate immune activation. Thus, the γ-secretase substrates that were identified in a microglia cell model seem to be recapitulated in one AD subtype only. Furthermore, Hou et al. notice that the effect of γ-secretase inhibition on microglial response to amyloid plaques was different from TREM2 knockout. The subtypes in our CSF proteomic data showed a similar dissociation: One subtype was enriched for TREM2 R47H and had a dampened microglial response; proteins increased in this subtype do not include those identified as a γ-secretase substrates in microglia.
Finally, the innate immune activation subtype had the highest levels of Bace1, Aβ40, and other markers of amyloid metabolism. The Hou et al. study suggests that, possibly, a microglial γ-secretase response may explain such high levels. This would be an interesting question to further investigate.
References:
Tijms BM, Vromen EM, Mjaavatten O, Holstege H, Reus LM, vanderLee SJ, Wesenhagen K, Lorenzini L, Vermunt L, Venkatraghavan V, Tesi N, Tomassen J, denBraber A, Goossens J, Vanmechelen E, Barkhof F, Pijnenburg YA, vanderFlier WM, Teunissen CE, Berven F, Visser PJ. Large-scale cerebrospinal fluid proteomic analysis in Alzheimer's disease patients reveals five molecular subtypes with distinct genetic risk profiles. 2023 May 11 10.1101/2023.05.10.23289793 (version 1) medRxiv.
Arkuda Therapeutics
In the past, analysis of γ-secretase (GS) substrates has been hampered by technical limitations. In this study, Hou et al. designed a very clever way of identifying novel substrates in an unbiased way. The failure of GSIs in the clinic was attributed in part to the impaired processing of Notch and potential ‘rebound’ Aβ production from APP (De Strooper, 2014), two of the most well characterized substrates of GS. With the identification of 85 substrates—59 novel—it is not at all surprising that GSIs had deleterious effects in patients.
The most remarkable finding is that the presence of amyloid plaques has such a dramatic effect on the disease-associated microglia (DAM) response gene signature following GS inhibition in vivo. Even though the plaque area was not different at 7 months, GS inhibition-induced reduction in the DAM gene expression profile suggests the microglia are less phagocytic. It is thus conceivable that at an older age, inhibition of GS in microglia would have led to plaque accumulation which, in the semagacestat clinical trial, could have also contributed to the worsening of patients.
While this study focuses on the microglial dysfunction caused by GS inhibition, the field would also benefit from future studies examining its consequence in neurons. We may never fully understand the complexity of the biology behind the deleterious effects of GSIs, but this study nicely provides a new hypothesis.
These results should however not let the field shy away from GS as a drug target. The recent approvals (Aduhelm, Leqembi) have shown that removing amyloid has a beneficial impact on disease progression. I believe now is time to think about γ-secretase modulators (GSMs) again and how they could be used in prevention trials (Bursavich et al., 2016).
References:
De Strooper B. Lessons from a failed γ-secretase Alzheimer trial. Cell. 2014 Nov 6;159(4):721-6. PubMed.
Bursavich MG, Harrison BA, Blain JF. Gamma Secretase Modulators: New Alzheimer's Drugs on the Horizon?. J Med Chem. 2016 Aug 25;59(16):7389-409. Epub 2016 Apr 5 PubMed.
University Kiel
The new study by Hou and coworkers in the lab of Bart de Strooper describes an interesting system-biology-based proteomics approach to unravel membrane substrates used by γ-secretase in cultured microglia cells. They found that at least 85 microglial membrane proteins are subject to γ-secretase-mediated processing.
The analysis is done under steady-state conditions, i.e., the exact sequential recognition and hydrolysis of substrates may have escaped detection. The authors also pinpoint the limitation that ectodomain shedding of membrane proteins as a prerequisite for most of the γ-secretase activity was not investigated. The problem of comparing cultured human microglia cells with microglia cells in vivo was mentioned as another limitation.
The in vivo studies presented in this study were focusing on murine microglia cells. Since γ-secretase may directly or indirectly modulate transcriptional activities, and since about two-thirds of the identified substrates are linked to microglia activation states, transcriptome profile analyses were performed. To this end, the used H9MG microglia cell line was “treated” with the approved γ-secretase inhibitor semagacestat and compared to vehicle-treated or LPS-treated cells. Gene set enrichment analyses after inhibitor treatment revealed about 350 genes, similar to the LPS-treated group, to be differentially regulated. This finding suggests that there is apparently a specific γ-secretase-dependent signature in microglia cells, which is related to the activation status of this cell type.
It would have been interesting to learn if the proteomes of treated versus control cells correspond to the transcriptional changes. It will also be interesting to study what determines the apparent differential use of transmembrane proteins by γ-secretase, and how the membrane cleavage may be linked to the observed transcriptome changes.
A transcriptome profile of microglial activation was also found in vivo in microglia, specifically in γ-secretase-deficient mice. When these mice were crossed with a murine AD model, the authors observed that amyloid plaque exposure apparently inhibited the development of a typical microglial transcriptional activation state. However, these γ-secretase-dependent changes were not found to cause an altered overall microglia morphology, distribution and handling of amyloid plaques.
An interesting aspect in this study is that semagacestat as a clinically relevant γ-secretase inhibitor causes, at least at the transcription level, significant regulatory responses in microglia cells in vitro and in vivo. Next to the largely unexplored consequences of the changed transcriptome profiles caused by γ-secretase inhibition, it will be fascinating to further explore the exact mechanisms of how γ-secretase modulators affect downstream signaling and nuclear transcriptional activities.
Sloan-Kettering Institute
De Strooper and colleagues examined the function of γ-secretase in microglia using both cellular and mouse models and offer exciting insights into γ-secretase’s action in microglia in response to amyloid insult.
γ-Secretase-mediated signal pathways play important roles in cell fate decisions of neurons, astrocytes, and oligodendrocytes in the nervous system (Louvi and Artavanis-Tsakonas, 2006) and in innate immunity (Liu et al., 2023). Conditional double knockout of PS1 and PS2, the catalytic subunits of γ-secretase, in the postnatal forebrain leads to impairments in memory and cognition (Saura et al., 2004). Hou et al. in this work, for the first time, reveal the role of γ-secretase in controlling the different states of microglia in response to amyloid and inflammation cues.
Although approximately 150 γ-secretase substrates have been reported (Güner and Lichtenthaler, 2020), systematic identification of γ-secretase substrates in different cell types is still a huge challenge in the field. An unbiased γ-secretase substrate identification (G-SECSI) platform developed in this work well addresses this issue, allowing the authors to analyze the function of γ-secretase and its substrates in a context-dependent manner.
γ-Secretase has been an attractive drug target due to its role in the production of Aβ peptides in neurons. This study shows that the inhibition or deletion of γ-secretase affects the function of microglia, reducing activity needed for Aβ clearance. It will be interesting to investigate how γ-secretase modulators, which selectively reduce γ-secretase production of Aβ42 offering an underlying mechanism for the development of γ-secretase-target based therapies (Crump et al., 2013), will regulate the activity of microglia.
References:
Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006 Feb;7(2):93-102. PubMed.
Liu C, Nikain C, Li YM. γ-Secretase fanning the fire of innate immunity. Biochem Soc Trans. 2023 Aug 31;51(4):1597-1610. PubMed.
Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004 Apr 8;42(1):23-36. PubMed.
Güner G, Lichtenthaler SF. The substrate repertoire of γ-secretase/presenilin. Semin Cell Dev Biol. 2020 Sep;105:27-42. Epub 2020 Jun 29 PubMed.
Crump CJ, Johnson DS, Li YM. Development and Mechanism of γ-Secretase Modulators for Alzheimer's Disease. Biochemistry. 2013 May 2; PubMed.
Make a Comment
To make a comment you must login or register.