Could New Alzheimer’s Marker p-Tau212 Rival p-Tau217?
Quick Links
Among plasma biomarkers for Alzheimer’s disease, p-Tau217 stands tall as having the highest diagnostic accuracy. Now, in the March 23 Nature Communications, researchers led by Przemyslaw Kac, Thomas Karikari, and Kaj Blennow at the University of Gothenburg, Mölndal, Sweden, report that a closely related phosphorylation site, p-Tau212, may work just as well. In tests on 388 participants in five cohorts, a plasma p-Tau212 immunoassay picked out people with AD as well as did p-Tau217, with an accuracy of 92 percent. In addition, a selective antibody against p-Tau212 lit up tangles and dystrophic neurites in postmortem brain samples as well as did one against p-Tau217, suggesting the two phospho-taus often occur together. However, in a memory clinic population, some patients had elevated plasma p-Tau212 alone, and others p-Tau217 alone, indicating that different pathological mechanisms trigger each phosphorylation.
- Plasma p-Tau212 identifies AD patients as well as does p-Tau217.
- The two markers agree 84 percent of the time, suggesting additive value.
- Both phospho-taus reflect similar brain pathology.
“That points to the importance of measuring more than one biomarker,” first author Kac told Alzforum. He believes p-Tau212 may be particularly relevant in preclinical AD and in Down’s syndrome; those studies are ongoing.
Suzanne Schindler at the University of Washington, St. Louis, said more work will be needed to determine the new marker’s usefulness. “Models that include both p-Tau212 and p-Tau217 as predictors would be helpful to determine whether p-Tau212 adds significant value to the more commonly measured p-Tau217,” she wrote to Alzforum (comment below).

New Contender. New p-Tau212 plasma assay correlates closely with plasma p-Tau217 as measured by mass spec, suggesting it could perform equally well as an AD diagnostic. [Courtesy of Kac et al., Nature Communications.]
In previous studies, p-Tau217 outperformed other fluid biomarkers, detecting amyloid plaques in the brain with 95 percent accuracy (Dec 2023 conference news). However, this is not high enough for a stand-alone clinical test, leading researchers to continue the hunt for additional markers (Nov 2023 conference news).
Kac and colleagues homed in on p-Tau212 because of its proximity and similarity to p-Tau217. Some kinases phosphorylate both sites, while others hit only one of them (Hanger et al., 2007; Liu et al., 2008; Tavares et al., 2013). To distinguish the two phospho-taus, the authors generated antibodies specific for each. In hippocampal and entorhinal cortical sections from two postmortem AD brains, both antibodies lit up neurofibrillary tangles, neuropil threads, and dystrophic neurites, indicating that the two p-Taus were equally present in pathological tau deposits. Likewise, immunofluorescent staining of temporal cortex sections from three AD brains showed almost identical patterns for the two (image below).
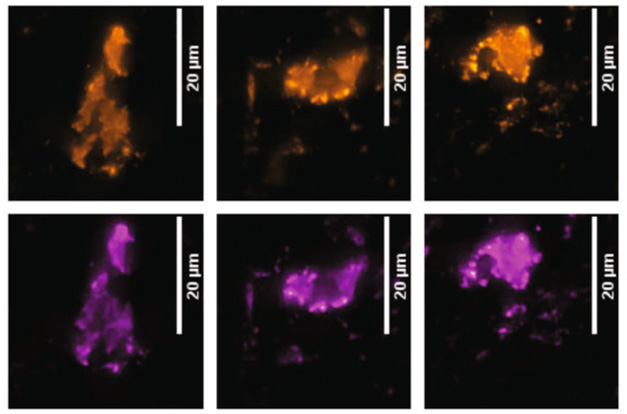
Same Signature. In temporal cortex samples from three AD brains (left to right), p-Tau217 (orange) and p-Tau212 (purple) light up tangles identically. [Courtesy of Kac et al., Nature Communications.]
Next, the authors developed an immunoassay for detecting p-Tau212 in body fluids. This Simoa assay can measure amounts as small as 0.17 pg/mL, a similar or slightly better sensitivity than most current p-Tau217 Simoa assays (Bayoumy et a., 2021).
Importantly, the assay performed equally well in plasma and cerebrospinal fluid. It distinguished 74 AD patients from 21 controls in a Polish cohort with comparable results in each type of fluid. The established biomarker p-Tau217 also works equally well in CSF and plasma, while other phospho-taus, such as p-Tau181 and p-Tau231, work much better in CSF (Palmqvist et al., 2020; Therriault et al., 2023). The results suggested that, like p-Tau217, p-Tau212 could make a promising plasma marker for clinical use.
Findings from a Gothenburg cohort bore this out. Plasma p-Tau212 distinguished 16 people with positive CSF AD biomarkers from 14 biomarker-negative controls with 92 percent accuracy. Notably, in both the Polish and Gothenburg cohorts, plasma p-Tau212, like p-Tau217, was four times higher in AD patients than controls, while p-Tau181 and p-Tau231 were only twice as high. A greater-fold difference makes for a more robust assay that is less affected by individual variations among people.
How would p-Tau212 fare in a real-world population? In a memory clinic cohort of 149 cognitively impaired people from Slovenia, plasma p-Tau212 performed as well as p-Tau217 to separate 103 amyloid-positive from 46 amyloid-negative people, with 93 to 96 percent accuracy. The two p-Tau markers agreed 84 percent of the time, again indicating that they reflected similar but not identical pathology.
The authors verified that p-Tau212 was detecting AD brain pathology using two neuropathology cohorts, the Baltimore Longitudinal Study of Aging and the University of California San Diego Neuropathology study. In the UCSD cohort, CSF p-Tau212 distinguished 40 AD cases from eight controls, as well as from 19 people with other pathologies. In the BLSA cohort, plasma p-Tau212 identified 20 autopsy-confirmed AD cases, but not 15 asymptomatic AD patients and 12 healthy controls.
In both neuropathology cohorts, the higher the load of plaques and tangles, the higher p-Tau212 was. However, the 15 asymptomatic cases belied this pattern, having a high pathology load but low p-Tau212 signal. Because this group stayed cognitively healthy, they probably represented people with high resilience to AD pathology, Kac noted. The low p-Tau212 in this group may indicate that this marker correlates with neuronal degeneration more than with pathology per se, he speculated.
In ongoing work, Kac is testing the p-Tau212 assay in preclinical AD populations. Preliminary findings suggest that p-Tau212 rises early in the disease, with higher-fold changes than other markers such as p-Tau181 and p-Tau231. In addition, because one of the kinases that phosphorylates threonine-212, DYRK1A, is located on chromosome 21, Kac believes p-Tau212 might be a particularly promising biomarker for people with Down’s syndrome. People with Down’s have three copies of chromosome 21, and develop AD pathology in middle age.—Madolyn Bowman Rogers
References
News Citations
- Two New p-Tau217 Blood Tests Join a Crowded Field
- Plasma p-Tau-217 Assays Work Well, But No Home Run for Diagnosis
Paper Citations
- Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007 Aug 10;282(32):23645-54. PubMed.
- Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, Ramakrishna N, Gong CX. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008 Sep;22(9):3224-33. PubMed.
- Tavares IA, Touma D, Lynham S, Troakes C, Schober M, Causevic M, Garg R, Noble W, Killick R, Bodi I, Hanger DP, Morris JD. Prostate-derived sterile 20-like kinases (PSKs/TAOKs) phosphorylate tau protein and are activated in tangle-bearing neurons in Alzheimer disease. J Biol Chem. 2013 May 24;288(21):15418-29. PubMed.
- Bayoumy S, Verberk IM, den Dulk B, Hussainali Z, Zwan M, van der Flier WM, Ashton NJ, Zetterberg H, Blennow K, Vanbrabant J, Stoops E, Vanmechelen E, Dage JL, Teunissen CE. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021 Dec 4;13(1):198. PubMed.
- Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, Su Y, Chen Y, Serrano GE, Leuzy A, Mattsson-Carlgren N, Strandberg O, Smith R, Villegas A, Sepulveda-Falla D, Chai X, Proctor NK, Beach TG, Blennow K, Dage JL, Reiman EM, Hansson O. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020 Aug 25;324(8):772-781. PubMed.
- Therriault J, Servaes S, Tissot C, Rahmouni N, Ashton NJ, Benedet AL, Karikari TK, Macedo AC, Lussier FZ, Stevenson J, Wang YT, Fernandez-Arias J, Stevenson A, Socualaya KQ, Haeger A, Nazneen T, Aumont É, Hosseini A, Rej S, Vitali P, Triana-Baltzer G, Kolb HC, Soucy JP, Pascoal TA, Gauthier S, Zetterberg H, Blennow K, Rosa-Neto P. Equivalence of plasma p-tau217 with cerebrospinal fluid in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2023 Apr 20; PubMed.
Further Reading
News
- p-Tau217 Immunotherapy Alleviates Tangle Pathology in Mice
- Paper Alert: Plasma P-tau217 Predicts Future Cognitive Decline
- Blood Tests Go Head-to-Head in Community Cohorts
- AD Blood Tests Are Here. Now, Let's Grapple With How to Use Them
- Paper Alert: p-Tau217 Blood Test Predicts Plaques, Tangles Over Time
Primary Papers
- Kac PR, González-Ortiz F, Emeršič A, Dulewicz M, Koutarapu S, Turton M, An Y, Smirnov D, Kulczyńska-Przybik A, Varma VR, Ashton NJ, Montoliu-Gaya L, Camporesi E, Winkel I, Paradowski B, Moghekar A, Troncoso JC, Lashley T, Brinkmalm G, Resnick SM, Mroczko B, Kvartsberg H, Gregorič Kramberger M, Hanrieder J, Čučnik S, Harrison P, Zetterberg H, Lewczuk P, Thambisetty M, Rot U, Galasko D, Blennow K, Karikari TK. Plasma p-tau212 antemortem diagnostic performance and prediction of autopsy verification of Alzheimer's disease neuropathology. Nat Commun. 2024 Mar 23;15(1):2615. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University
Kac and colleagues validated an immunoassay for plasma p-Tau212 in multiple clinical and autopsy cohorts. They demonstrated that their p-Tau212 antibody does not cross-react with p-Tau217. However, the results for their plasma p-Tau212 and p-Tau217 assays are very similar. Notably, differences in assay performance unrelated to the assay target (p-Tau212 versus p-Tau217) could account for small differences in the results. Models that include both p-Tau212 and p-Tau217 as predictors would be helpful to determine whether p-Tau212 adds significant value to the more commonly measured p-Tau217.
Make a Comment
To make a comment you must login or register.