Diabetes Drug May Rev Up Brain Metabolism in People with Alzheimer’s
Quick Links
Brain metabolism wanes as Alzheimer’s disease worsens, but results from a small trial hint that a diabetes drug may halt the slow-down. Researchers led by Jørgen Rungby at Aarhus University in Denmark reported that AD patients treated with liraglutide, an analogue of the hormone glucagon-like peptide 1 (GLP-1), were spared from a drop in cerebral glucose metabolism over six months. Whether the metabolic boost altered amyloid burden or cognition was unclear due to the small number of patients enrolled in the study, but larger ongoing trials may soon yield some answers. The whiff of positive results from this proof-of-concept study, published online in Frontiers in Aging Neuroscience on April 26, indicate that researchers should look deeper into this group of drugs, Rungby told Alzforum.
Thomas Foltynie of University College London, who is investigating the use of GLP-1 analogues to treat Parkinson’s disease, agreed, despite the limitations of the study. “On balance, this study provides further encouragement to those investigating whether this class of drugs may have potentially important beneficial effects in a range of neurodegenerative processes in the brain.”
GLP-1 analogues stimulate insulin signaling, which promotes the uptake and utilization of glucose. These compounds effectively stimulate glucose metabolism in people with diabetes. However, the drugs also exert a range of effects in the brain. In animal models, GLP-1 analogues promoted neuronal survival, boosted synaptic function, inhibited neuroinflammation, reduced amyloid plaque formation, and supported learning and memory (see Hansen et al., 2015; Perry et al., 2003; and Aug 2003 news). The drugs have been reported to improve brain glucose transport in people, and some scientists believe GLP-1 analogues look promising as drugs to fend off neurodegeneration (see Gejl et al., 2013; Dec 2010 conference news).
For the current double-blind, randomized trial, first author Michael Gejl and colleagues recruited 38 people with AD and randomized them to take liraglutide or placebo. Fourteen people in the liraglutide group and 20 people in the placebo group completed the trial. The researchers used PET scans to measure cerebral glucose metabolism (using an FDG tracer) and amyloid burden (using PiB), and also tested the participants for cognitive changes using the WMS-IV scale, before and after the treatment period.
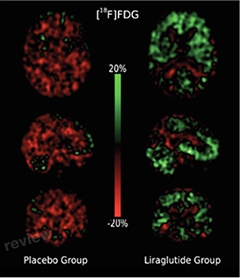
Only cerebral glucose metabolism displayed a significant difference between the placebo and treatment groups. Glucose metabolism held steady over the six-month trial period in AD patients who took liraglutide, while their counterparts in the placebo group displayed a significant reduction in metabolism across several brain regions. Amyloid load increased and cognition remained unchanged in both groups.
Despite the exceedingly small numbers of patients in the trial, the glucose metabolism results were surprisingly clear and impressive, commented Christian Holscher of Lancaster University in England. “The FDG PET biomarker correlates well with brain activity, function, and disease progression,” he added, “so if you see a change in that parameter, it really means something.”
The short duration of the trial as well as the inclusion of patients who already had significant amyloid deposition and cognitive deficits made it unlikely that a benefit would have been observed in those outcomes, commented Dimitrios Kapogiannis of the National Institute on Aging in Bethesda, Maryland. “Still, the fact that the researchers saw an effect on glucose metabolism was encouraging,” he added.
Researchers will have to wait on the results of larger ongoing trials before drawing conclusions about the effect of GLP-1 analogues on amyloid load or cognition, Holscher said. One such trial, led by Paul Edison at Imperial College London, is measuring liraglutide’s effects on brain glucose metabolism as well as CSF biomarkers in more than 200 people with early AD over one year. Kapogiannis is conducting a similar trial using exendin-4, another GLP-1 analogue. Ongoing trials in Parkinson’s will shed light on the general regenerative value of GLP-1 analogues, researchers agreed.
Preclinical studies have revealed that GLP-1 analogues act in multiple ways, ranging from anti-inflammatory to neuroprotective to amyloid-reducing, commented Nigel Greig of the National Institute on Aging. “By hitting a number of targets, it allows you to hopefully hit something that’s relevant for each disease at different stages,” he said. For example, while people with AD who already have substantial brain amyloid may not experience amyloid reduction, they may still benefit from the drug’s neuroprotective effects.
The researchers added that neither Novo Nordisk (the makers of liraglutide) or Bristol-Myers Squibb (the makers of exenetide, another GLP-1 analogue) have expressed interest in sponsoring a Phase 3 study should the Phase 2 studies pan out. However, Rungby added that because the drugs already have been approved for diabetes, future trials for neurodegenerative disease could be streamlined.—Jessica Shugart
References
News Citations
- Learning and Neuroprotective Role Proffered for Glucagon-Derived Peptide
- San Diego: A New Tack on Insulin-Based Therapies?
Paper Citations
- Hansen HH, Fabricius K, Barkholt P, Niehoff ML, Morley JE, Jelsing J, Pyke C, Knudsen LB, Farr SA, Vrang N. The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2015 Jun 26;46(4):877-88. PubMed.
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003 Jun 1;72(5):603-12. PubMed.
- Gejl M, Lerche S, Egefjord L, Brock B, Møller N, Vang K, Rodell AB, Bibby BM, Holst JJ, Rungby J, Gjedde A. Glucagon-like peptide-1 (GLP-1) raises blood-brain glucose transfer capacity and hexokinase activity in human brain. Front Neuroenergetics. 2013;5:2. Epub 2013 Mar 27 PubMed.
External Citations
Further Reading
Papers
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. Drug Discov Today. 2016 May;21(5):802-18. Epub 2016 Feb 3 PubMed.
- Greig NH, Tweedie D, Rachmany L, Li Y, Rubovitch V, Schreiber S, Chiang YH, Hoffer BJ, Miller J, Lahiri DK, Sambamurti K, Becker RE, Pick CG. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014 Feb;10(1 Suppl):S62-75. PubMed.
Primary Papers
- Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht A, Møller N, Brock B, Rungby J. In Alzheimer's Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front Aging Neurosci. 2016;8:108. Epub 2016 May 24 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCL Institute of Neurology
This is a small, short-term but exciting study investigating the possible effects of Liraglutide on disease progression in Alzheimer’s. The headline findings are that patients on the active drug had a cessation in the decline of glucose metabolism in the brain as measured by [18F]FDG PET. This is of considerable potential relevance given that decline in this measure has previously been strongly linked to cognitive decline.
The authors are correct to be cautious about the clinical results they observed given the small sample size, chance baseline differences in age and disease duration between the groups, and the failure to achieve conventional levels for statistical significance, but it is encouraging that the overall decline in cognitive performance was also slightly smaller in the Liraglutide treatment group than the placebo group. The drug seemed to be well-tolerated although weight loss (mean 4.9Kg) may be an issue for some patients.
The study did, however, fail to find any difference in the primary outcome measure of amyloid deposition in the brain as measured by [11C] PiB PET binding. Whether this relates to the duration of exposure, stage of disease, or simply reflects insufficient efficacy of the drug remains to be seen.
On balance, this study provides further encouragement to those investigating whether this class of drugs may have potentially important beneficial effects in a range of neurodegenerative processes in the brain.
Make a Comment
To make a comment you must login or register.