Do Tribes of Astrocytes Wage War on Synapses?
Quick Links
As researchers are becoming wise to microglial diversity, they may also want to keep their eyes out for varied populations of the true glia of the brain. In the April 1 Nature Neuroscience, Jeffrey Rothstein’s group at Johns Hopkins University in Baltimore report a type of astrocyte with a distinct distribution and molecular profile. Residing mainly in layer V of mouse cortex, these cells produce Norrin, which regulates spine density and synaptic function in surrounding neurons. Mutations in Norrin cause Norrie disease, a developmental disorder.
- Scientists identify a special tribe of astrocytes in layer V of the brain’s cortex.
- The cells tweak synapses by spilling Norrin, linked to Norrie disease.
- Are there more disease-related astroglia, waiting to be discovered?
“One major implication of this work is that other neurodevelopmental/degenerative disorders could arise from a similar disruption between astrocytes and neurons,” wrote Christopher Norris, University of Kentucky, Lexington. In Alzheimer’s disease (AD), spine loss and synaptic dysfunction directly correlate with dementia. “This exciting new study reinforces the mechanistic importance of astrocytes to synapse vitality, and the potential of astrocyte-specific targets for treating synapse dysfunction and cognitive loss in neurodegenerative diseases like AD,” he wrote to Alzforum.
Single-cell transcriptomics has focused attention on microglial diversity (Feb 2019 news; Dec 2018 news), but a similar analysis of astrocytes has lagged behind. Like microglia, astrocytes play multiple roles in the brain, ensuring proper levels of extracellular ions, clearing excess neurotransmitters, controlling blood flow to active neurons, and aiding synapse formation and renewal. They also participate in amyloid- and tau-induced synapse loss (Jan 2017 news; Sep 2017 news). While scientists have long recognized morphologically distinct astrocyte subsets, the study of their molecular and functional diversity is in its infancy (Haim and Rowitch, 2017). Only a few studies have analyzed astrocytes with single-cell RNA sequencing, for example (Tasic et al., 2016; Darmanis et al., 2015; Ziesel et al., 2015).
In the new study, first author Sean Miller set out to explore cell-specific regulation of the expression of the astrocyte glutamate transporter 1 (Glt1 or EAAT2). Glt1 levels are highest in astrocytes of the hippocampus and cortex, lowest in the spinal cord, and dysregulated in amyotrophic lateral sclerosis (ALS). By analyzing Glt1 promoter activity in mice expressing a red fluorescent reporter, Miller found an 8.3 kilobase fragment that was only active in a subset of gray-matter astrocytes restricted to cortical layer V.
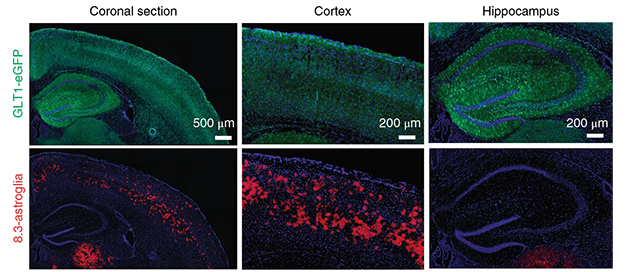
Select Few. In a sea of cortical astroglia (green cells in top panels), an 8.3 kB Glt1 promoter fragment is active in only a subset, where it drives expression of a red fluorescent reporter (bottom panels). No 8.3-astroglia are found in the hippocampus. [Courtesy of Miller et al., 2019 Nature Neuroscience.]
Transcriptome profiling revealed that the 8.3-astroglia were marked by elevated expression of several proteins, including Norrin, OLIG2, KCNJ10, and the receptor LGR6, which was 10-fold higher on these astroglia. The investigators also detected LGR6-positive astrocytes in human postmortem brain tissue.
The LGR6 astroglia played a role in maintaining normal dendritic spines in the layer V neurons. Miller found that neurons produced the LGR6 ligand, R-spondin1, which increased proliferation of the astrocytes and boosted their Norrin output. On primary mouse cortical neurons, Norrin made their dendrites longer and more arborized, and strengthened the firing and connectivity of their synapses.

Branching Out. In cultured cortical neurons, Norrin (right) made dendrites longer and more branched. [Courtesy of Miller et al., 2019 Nature Neuroscience.]
Mutations in Norrin cause Norrie disease. This X-linked neurodevelopmental condition primarily affects sight and hearing, but also can cause mental retardation and psychosis and even, in rare cases, dementia in early adulthood. Norrin knockout mice had 50 percent fewer dendritic spines in cortical layer V. They appeared hyperactive, resting less, running faster, and rearing up on their hind legs more than wild-type mice. When Miller expressed Norrin specifically in the 8.3-astroglia in either Norrin or LGR6 knockout mice, their spine density was partially restored. Taken together, the results indicate that that loss of Norrin expression by 8.3-astroglia may underlie some of the symptoms of Norrie disease.
Does Norrin have any bearing on Alzheimer’s disease? Tara-Spires Jones, University of Edinburgh, thinks it is worth a look. “I haven’t seen any papers linking Norrin to synapse integrity, so this is a novel pathway for the AD community to investigate,” she wrote to Alzforum. Previous work from Elly Hol’s group at the Brain Center at University Medical Center, Utrecht, in The Netherlands, noted a drop in Norrin expression in cortical astrocytes in AD mouse models (Orre et al., 2014). Hol told Alzforum they had not looked at spine loss in those mice, but with this new work they will. Notably, Rothstein’s group detected no 8.3-astroglia in the hippocampus, where early spine loss occurs in AD.
“This was an awesome detective story,” said Philip Haydon, Tufts University, Boston. “These researchers did a superb job of linking together signals from neuron to astrocytes and back again, and showing the importance of Norrin in spine density—it’s a tour de force,” he told Alzforum. “I think this work will help shine some light back on astrocytes,” he said.—Pat McCaffrey
References
News Citations
- Single-Cell Profiling Maps Human Microglial Diversity, Flexibility
- Microglia Reveal Formidable Complexity, Deep Culpability in AD
- Microglia Give Astrocytes License to Kill
- ApoE4 Makes All Things Tau Worse, From Beginning to End
Paper Citations
- Ben Haim L, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017 Jan;18(1):31-41. Epub 2016 Dec 1 PubMed.
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, Bertagnolli D, Goldy J, Shapovalova N, Parry S, Lee C, Smith K, Bernard A, Madisen L, Sunkin SM, Hawrylycz M, Koch C, Zeng H. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016 Feb;19(2):335-46. Epub 2016 Jan 4 PubMed.
- Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, Hayden Gephart MG, Barres BA, Quake SR. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7285-90. Epub 2015 May 18 PubMed.
- Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015 Mar 6;347(6226):1138-42. Epub 2015 Feb 19 PubMed.
- Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, Hol EM. Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction. Neurobiol Aging. 2014 Dec;35(12):2746-60. Epub 2014 Jun 14 PubMed.
Further Reading
Papers
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015 Jul;18(7):942-52. PubMed.
Primary Papers
- Miller SJ, Philips T, Kim N, Dastgheyb R, Chen Z, Hsieh YC, Daigle JG, Datta M, Chew J, Vidensky S, Pham JT, Hughes EG, Robinson MB, Sattler R, Tomer R, Suk JS, Bergles DE, Haughey N, Pletnikov M, Hanes J, Rothstein JD. Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin. Nat Neurosci. 2019 Apr 1; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Kentucky College of Medicine
Historically, neurodegeneration research has been dominated by a neuron-centric focus. This has changed somewhat with the increasing recognition that glial cells mediate numerous protective and detrimental immune/inflammatory processes found in most neurodegenerative diseases. Among the different glial cell types, astrocytes have frequently played “second fiddle” to microglia, which are widely thought of as the resident immune cells of the CNS. But the importance of astrocytes, and the possibility that these cells host possible drug targets for neurodegenerative diseases, like Alzheimer’s disease (AD), is gaining steam.
Like good real estate, the gaining appeal of astrocytes has a lot to do with location: Astrocyte processes ensheathe the cerebrovasculature as well as many, if not most, excitatory synaptic connections. In this regard, astrocytes are ideally positioned to contribute to pathological changes in blood vessels and synaptic integrity, which are found early in the progression of many neurodegenerative diseases. Astrocytes are well-known to exhibit key metabolic functions in the brain. And through the uptake of glutamate and K+ ions, astrocytes provide an essential defense against excitotoxic damage to synapses and dendrites. Astrocytes also secrete a number of factors, such as matricellular proteins and complement components, that directly regulate the maintenance or turnover of synapses. Thus, several recent studies have suggested that astrocyte activation and/or dysfunction lead to synapse loss in Alzheimer’s disease (Furman et al., 2012; Hefendehl et al., 2016; Sompol et al., 2017; Liddelow et al., 2017; Shi et al., 2017; Hong et al., 2016).
The recent article by Miller et al. significantly advances this line of research by showing that synapse loss/dysfunction in discrete brain regions may be directly mediated by very specific subpopulations of astrocytes. Through an extensive series of studies combining newly developed transgenic mice with transcriptomic and immunohistochemical approaches, the authors convincingly reveal the existence of a distinct subpopulation of gray-matter astrocytes in the neocortex (particularly in Layer V), referred to as “8.3-astrocytes.” These astrocytes exhibit a unique transcriptional signature, highlighted by the expression of Lgr6 (which encodes a G-protein coupled receptor) and Norrin (which encodes a secreted protein that triggers Wnt signaling and neurotrophic factor production). Importantly, this subpopulation of astrocytes was also identified in human neocortex. Additional studies showed that neurons in 8.3-astrocyte-enriched regions expressed uniquely elevated levels of the Lgr6 ligand, RSPO1. Stimulation of primary astrocytes with RSP01 triggered the Lgr6-dependent release of Norrin. Moreover, modulation of Norrin levels caused alterations in dendritic spine density and dendrite morphology (i.e., loss of Norrin led to reduced spine density), along with neurobehavioral changes (e.g., hyperactivity). The authors suggested that neurodevelopmental disorders characterized by dendritic spine loss, such as Norrie disease, may be directly attributable to dysfunctional interactions between 8.3-astrocytes and surrounding cortical neurons.
One of the major implications of this work is that other neurodevelopmental/degenerative disorders could arise from a similar disruption between astrocytes and neurons. So, while 8.3-astrocytes and Norrin may not play a specific role in Alzheimer’s disease—in fact, the authors showed that 8.3-astrocytes were completely absent in the hippocampus, one of the structures that show early synapse loss in AD—it’s possible that a yet-to-be-defined astrocyte subpopulation leads to synapse loss and/or vulnerability in select brain regions with AD. An intriguing possibility is that the unique temporal and spatial propagation of AD pathologies fundamentally relates to abnormal interactions between neurons and specific subpopulations of astrocytes. In any case, the results of this exciting new study reinforce the mechanistic importance of astrocytes to synapse vitality and the potential of astrocyte-specific targets for treating synapse dysfunction and cognitive loss in neurodegenerative diseases like AD.
References:
Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J Neurosci. 2012 Nov 14;32(46):16129-40. PubMed.
Hefendehl JK, LeDue J, Ko RW, Mahler J, Murphy TH, MacVicar BA. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat Commun. 2016 Nov 11;7:13441. PubMed.
Sompol P, Furman JL, Pleiss MM, Kraner SD, Artiushin IA, Batten SR, Quintero JE, Simmerman LA, Beckett TL, Lovell MA, Murphy MP, Gerhardt GA, Norris CM. Calcineurin/NFAT Signaling in Activated Astrocytes Drives Network Hyperexcitability in Aβ-Bearing Mice. J Neurosci. 2017 Jun 21;37(25):6132-6148. Epub 2017 May 30 PubMed.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan 26;541(7638):481-487. Epub 2017 Jan 18 PubMed.
Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, Stevens B, Lemere CA. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med. 2017 May 31;9(392) PubMed.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016 May 6;352(6286):712-6. Epub 2016 Mar 31 PubMed.
Duke University
This study provides insights for addressing two important questions, the first of which is whether unique subgroups of astrocytes exist in the mammalian cortex. Evidence exists to support astrocyte heterogeneity between brain regions, and astrocytes are known to differentially respond in various diseases, but less is known regarding subgroups of astrocytes in the healthy cortex. This study identifies a novel, reproducibly identifiable subclass of cortical astroglia (8.3-astroglia) whose transcriptomic and proteomic profile distinguishes it from neighboring astrocytes.
Secondly, this study provides an attractive possible mechanism through which 8.3-astroglia participate in dendrite/spine formation by secreting Norrin, a protein that is linked to Norrie disease. The identification of such a mechanism is important for our general understanding of how astrocytes contribute to neuronal and synaptic development in the healthy brain, and have important implications for the understanding of Norrie disease. Even though this work does not have direct links to AD pathophysiology, it provides important evidence putting forth astrocyte dysfunction as an underlying mechanism in synaptic pathologies seen in neurological disorders.
University of Toronto
Regarding aging-related tau astrogliopathy, there is no clear-cut selective involvement of the fifth cortical layer—in my experience.
However, I find this report extremely exciting. Since the morphology of pathological tau accumulation can be dramatically different between disease entities of tauopathies, it has been considered that perhaps this means that there are different subtypes of astroglia, which then generate different morphological forms of tau. This concept awaited research results from basic science confirming or suggesting that indeed there are different astroglial subtypes. So, on a conceptual level I find this paper very important as a pioneering step toward defining astroglia populations—even if at this moment I cannot associate the present findings with patterns of tau pathology.
University of Edinburgh
The finding by Miller et al. that a subset of mouse cortical astrocytes secrete Norrin, which is important for dendritic spine integrity, is very interesting. Synapse loss is the strongest pathological correlate of cognitive decline in Alzheimer’s disease, and it is becoming clear that glia modulate synapse degeneration (Henstridge et al., 2019). A lot of progress has been made recently in understanding age, brain region, and disease effects on microglial heterogeneity and how this might impact upon synapse degeneration in AD (see for example Grabert et al., 2016; Hong et al., 2016; Keren-Shaul et al., 2017; Patir et al., 2019). Less work has been done on astrocyte heterogeneity but astrocytes have also been seen to exhibit region-specific, age-associated changes that are influenced by microglial phenotypes (Clarke et al., 2018).
Recent data suggest that astrocytes are important for synaptic pruning in both microglial-dependent and independent pathways (Chung et al., 2016; Liddelow et al., 2017). I haven’t seen any papers before this one linking Norrie to synapse integrity, so this is a novel pathway for the AD community to investigate. The more we can piece together the complex interactions between cell types in this important pathological phenomenon, the better chance we have of finding an effective way to prevent or reverse synapse loss in AD.
References:
Chung WS, Verghese PB, Chakraborty C, Joung J, Hyman BT, Ulrich JD, Holtzman DM, Barres BA. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):10186-91. Epub 2016 Aug 24 PubMed.
Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):E1896-E1905. Epub 2018 Feb 7 PubMed.
Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016 Mar;19(3):504-16. Epub 2016 Jan 18 PubMed.
Henstridge CM, Hyman BT, Spires-Jones TL. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci. 2019 Feb;20(2):94-108. PubMed.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016 May 6;352(6286):712-6. Epub 2016 Mar 31 PubMed.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017 Jun 15;169(7):1276-1290.e17. Epub 2017 Jun 8 PubMed.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan 26;541(7638):481-487. Epub 2017 Jan 18 PubMed.
Patir A, Shih B, McColl BW, Freeman TC. A core transcriptional signature of human microglia: Derivation and utility in describing region-dependent alterations associated with Alzheimer's disease. Glia. 2019 Jul;67(7):1240-1253. Epub 2019 Feb 13 PubMed.
VU University Medical Center
This is interesting data on the function and expression of Norrin (NDP) in the brain. Thus far limited research has been performed on the role of this protein and the effects of loss-of-function mutations on the brain. Especially data on human brain tissue is largely missing for both Norrie disease and in relation to neurodegeneration.
We recently published a paper (Hondius et al., 2018) that indicated the relevance of Norrin at the protein level in a subtype of Alzheimer’s disease. In this study, we performed a proteomics analysis on laser-dissected human brain tissue. Control cases were compared with cases that have end-stage AD with severe Aβ plaque pathology and cases that exhibit a severe vascular form of AD, specifically cerebral amyloid angiopathy (CAA) type-1. In this type of disease, Aβ accumulates around the brain capillaries and larger vessels.
We found that the expression of Norrin protein was increased only in cases that exhibit CAA type-1 but not in AD cases with only Aβ plaques. Immunohistochemistry revealed a large increase in Norrin immunoreactivity and localization to CAA-affected vessels, especially capillaries, but not to Aβ plaques. Interestingly, a similar increase was found in prion-CAA, where capillaries were affected. Moderate immunoreactivity was found in a CADASIL case related to affected vessels in the white matter.
We proposed Norrin to be exploited as a biomarker to effectively distinguish AD subtypes based on the presence and amount of vascular-related pathology. In addition, Norrin might offer therapeutic possibilities, as we learn more on the role of Norrin in neurodegeneration, especially with involvement of the brain vasculature.
The cellular origin of the increased levels of Norrin in CAA remains largely unclear, as well as the direct consequence of this increase with respect to synaptic function, blood-brain barrier function and disease progression.
References:
Hondius DC, Eigenhuis KN, Morrema TH, van der Schors RC, van Nierop P, Bugiani M, Li KW, Hoozemans JJ, Smit AB, Rozemuller AJ. Proteomics analysis identifies new markers associated with capillary cerebral amyloid angiopathy in Alzheimer's disease. Acta Neuropathol Commun. 2018 Jun 4;6(1):46. PubMed.
Make a Comment
To make a comment you must login or register.