Does Second X Chromosome Boost Women’s Resilience Against Alzheimer’s?
Quick Links
As a group, women may be more likely than men to develop Alzheimer’s disease, but once they have it, they cope better and live longer. A study published August 26 in Science Translational Medicine credits a second X chromosome with some of that resilience—at least in mice. “This paper is an exciting and novel examination of the mechanisms that underlie sex differences in AD risk/resilience,” commented Rachel Buckley of Massachusetts General Hospital in Boston.
- Female hAPP mice outlive males.
- Two X chromosomes protect mice, regardless of a Y, or gonads.
- The gene Kdm6a, which evades X inactivation, partly explains the resilience.
- Variants that boost KDM6A expression confer resilience to AD.
Scientists led by Dena Dubal at the University of California, San Francisco, studied a menagerie of mice with X, XX, XY, and XXY chromosomes, some of whom lacked gonads to avoid complications of sex hormones. The scientists found that two X chromosomes made for a longer life with less memory loss in mice with amyloidosis. The researchers pinned part of the protection on Kdm6a, an X chromosome gene that encodes a histone demethylase. Kdm6a evades X-inactivation, a process whereby most of the genes on one of a female’s two X chromosomes get silenced. Separately, the researchers found that people who carry a genetic variation that boosts expression of the gene slide more slowly on cognitive tests during aging and in preclinical AD.
The findings underscore the importance of genes on the X chromosome—typically overlooked in genetic studies—in shaping resilience to AD, Dubal told Alzforum. A mechanistic understanding of resilience may even point to therapeutic strategies that could benefit people regardless of how many Xs they have, she said.
The time between diagnosis and death varies markedly among people who get AD, ranging from five to 20 years (Jun 2020 news). Some people even remain cognitively normal for some years despite having large amounts of amyloid plaques and neurofibrillary tangles in their brains. What explains this resilience? Genetic studies are starting to uncover variants that boost resilience (Jul 2020 conference news).
Sex could play a role, too, as some studies suggest that men with AD succumb to the disease faster than women, or that men suffer more neurodegeneration and cognitive decline than women with a similar tangle burden (Lapane et al., 2001; Ossenkoppele et al., 2020; Digma et al., 2020). The literature is mixed, however, with some studies suggesting that women decline faster than men, especially in the later stages of AD (Aug 2018 conference news; Buckley et al., 2018).
Reasoning that sex differences could uncover novel mechanisms of resilience, co-first authors Emily Davis and Lauren Broestl started with a meta-analysis to confirm that there is a relationship between sex and AD duration. They integrated data from 16 longitudinal studies that measured the duration of disease after onset, and found that, overall, men died 62 percent sooner than women with the disease.
Similarly, in the hAPP mouse model of amyloidosis, males died earlier than females. This was true even when the researchers removed the ovaries or testes from the mice at 10 weeks of age, suggesting that this difference was not due to circulating sex hormones. In addition to dying young, male hAPP mice were more impaired than females on learning and memory tests despite having similar levels of plaques, soluble Aβ, and phospho-tau in the hippocampus. After 2 years of age, females had slightly more plaques than males.
How did female hAPP mice outperform and outlast their male counterparts? Davis and colleagues focused on the role of sex chromosomes. First, the researchers used the Four Core Genotypes (FCG) mouse model, in which the Sry gene is deleted from its typical location on the Y chromosome. Sry dictates development of the testes and male characteristics. The researchers moved the Sry gene to an autosomal location in some of the mice; this maneuver allowed them to control the development of male sex independently of the Y chromosome. They generated four sex genotypes: XX or XY, each with ovaries, i.e., -Sry, or testes, i.e. +Sry. The researchers generated hAPP mice with all four sex genotypes. They removed their gonads at around 3 months of age to remove the influence of sex hormones beyond development.
They found that hAPP mice with two X chromosomes lived longer than their XY counterparts, regardless of whether they expressed Sry. Similarly, XX-hAPP mice had better memory than XYs, regardless of whether they initially developed ovaries or testes.
Mouse Gender Jungle
Did XY, i.e., typical male hAPP mice flounder due to the presence of their Y, or absence of a second X? To answer this question, the researchers crossed hAPP mice to yet another gender model, XY* mice, which arise from a recombination snafu during meiosis. Offspring of XY* males and XX females produce four sex genotypes: XX and XO mice, both with ovaries, and XY and XXY mice, both with testes. XO mice have only a single sex chromosome. The researchers found that regardless of the presence of a Y chromosome, ovaries, or testes, hAPP mice with two X chromosomes, including the XXYs, lived longer and performed better on memory tests than mice with a single X, i.e., the XOs and XYs. The findings suggested that having two X chromosomes, as opposed to lacking a Y, conferred resilience to Aβ pathology.
But how does that second X chromosome do that? In female mammalian cells, X-inactivation silences most genes on one of the X chromosomes to ensure their expression is not doubled. However, a few genes manage to elude this epigenetic crackdown, riffing off transcripts from both Xs. One such gene—in both mice and people—is Kdm6a (Greenfield et al., 1998). Also called Utx, it encodes a histone demethylase, enzymes that open up chromatin to activate transcription. People with loss-of-function mutations in the gene develop Kabuki syndrome, a disorder that causes intellectual disability. Mice without the gene have deficits in synaptic plasticity and memory (Miyake et al., 2013; Tang et al., 2017).
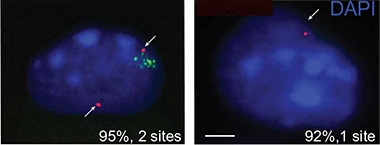
Double Dose. KDM6A RNA (red) is transcribed from two sites in XX neuronal nuclei (left), despite X-inactivation (green). Only one site transcribes the gene in XY nuclei (right). [Courtesy of Davis et al., Science Translational Medicine, 2020.]
The researchers confirmed that KDM6A was transcribed from both X chromosomes in female mouse neurons, and that the female hippocampus had 30-50 percent more of the mRNA and protein than did the male hippocampus. In subsequent series of experiments, the researchers found that boosting KDM6A expression in the brains of male hAPP mice relieved memory deficits. Overexpressing KDM6A in primary neurons from male mice shielded the cells from Aβ oligomer toxicity, while dampening its expression in female neurons rendered the cells more vulnerable. In all, the data suggested that, in the mouse brain, KDM6A explained part of the female resilience to Aβ pathology, and that raising its expression could protect neurons from it.
To check whether this could be relevant in the human brain, the scientists consulted three public datasets that catalog gene expression in postmortem brain samples. From a total of more than 500 subjects, they found higher expression of KDM6A in the temporal cortex, parahippocampal gyrus, and superior temporal gyrus in people with AD than in controls. KDM6A expression did not differ between cases and controls in brain regions typically spared in AD, such as the cerebellum. Dubal hypothesized that this increased expression in AD brain regions could reflect a compensatory response. In keeping with their double dose of the gene, KDM6A expression was also higher in women, with or without AD.
Besides the doubling of expression due to a second copy of the gene, there could be other types of genetic variation that boost KDM6A expression. To check for this, the researchers turned to the Genotype-Tissue Expression (GTEX) project, an online portal with gene expression and genome sequencing data on tissues from nearly 1,000 people. They searched for variants associated with elevated KDM6A expression in the brain, and, sure enough, identified a common variant, rs12845057, whose minor allele, an adenosine, is carried by roughly 14 percent of the global population and associates with more KDM6A expression in the brain.
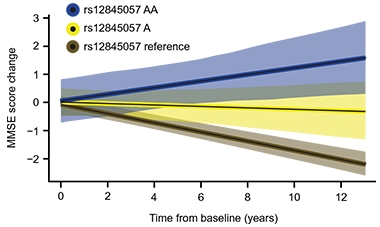
Resilience Variant. Carriers of two (blue) or one (yellow) copy of a variant that boosts KDM6A expression decline more slowly on the mini mental state exam than noncarriers (brown). [Courtesy of Davis et al., Science Translational Medicine, 2020.]
To see if this variant associated with cognitive resilience, the researchers looked for it among 778 volunteers in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). The KDM6A minor allele was equally distributed among cognitively normal people, those with mild cognitive impairment, and AD, suggesting it did not alter a person’s risk of getting AD. But when considering men and women combined, the researchers found that 78 people with one copy of the allele declined more slowly on the mini mental state examination (MMSE) than did 692 noncarriers. The eight women in this sample who had two copies of the variant even seemed to improve their scores over time (see image above). When the researchers limited their analysis to men, the allele had no effect. Dubal said that this is likely due to lack of statistical power, exacerbated by the fact that men can only carry a single copy of the gene.
Dubal’s lab is investigating how KDM6A protects cultured neurons from Aβ toxicity, and whether the protein shields against insults from tau, α-synuclein, ApoE4, and age-related cellular stress. She plans to investigate whether other X-inactivation escapees in the human brain are also involved in resilience. “If we understand what makes one sex more resilient than another, then maybe we can harvest that knowledge and apply it to both sexes,” she said.
Buckley noted that KDM6A’s escape from X-inactivation is likely to have different consequences in different cell types, given that its expression in T cells was linked to autoimmunity in women (Itoh et al., 2019). “This won’t be a one-size-fits-all risk gene,” she cautioned.
Why has KDM6A not turned up in the large genetic association studies of AD? Michelle Mielke of the Mayo Clinic in Rochester, Minnesota, noted that most GWAS have historically excluded the sex chromosomes. “This study highlights the need to examine genes on the X and Y chromosomes for risk and resiliency to AD and other dementias,” Mielke wrote. She added that while the study clearly establishes a role for sex chromosomes, as opposed to hormones, in resilience in the hAPP mouse model, it does not examine the potential influence of hormonal treatments on this resilience.—Jessica Shugart
References
News Citations
- Tau: Why Alzheimer’s Worsens Fast in Some, Slowly in Others
- Doubling Down on Sequencing Serves up More Alzheimer’s Genes
- Do Brain Changes at Menopause Make Women More Prone to Alzheimer’s?
Research Models Citations
Paper Citations
- Lapane KL, Gambassi G, Landi F, Sgadari A, Mor V, Bernabei R. Gender differences in predictors of mortality in nursing home residents with AD. Neurology. 2001 Mar 13;56(5):650-4. PubMed.
- Ossenkoppele R, Lyoo CH, Jester-Broms J, Sudre CH, Cho H, Ryu YH, Choi JY, Smith R, Strandberg O, Palmqvist S, Kramer J, Boxer AL, Gorno-Tempini ML, Miller BL, La Joie R, Rabinovici GD, Hansson O. Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurol. 2020 May 1;77(5):632-642. PubMed.
- Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ, Alzheimer’s Disease Neuroimaging Initiative. Women can bear a bigger burden: ante- and post-mortem evidence for reserve in the face of tau. Brain Commun. 2020;2(1):fcaa025. Epub 2020 Apr 14 PubMed.
- Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs HI, Burnham S, Hanseeuw BJ, Doré V, Dobson A, Masters CL, Waller M, Rowe CC, Maruff P, Donohue MC, Rentz DM, Kirn D, Hedden T, Chhatwal J, Schultz AP, Johnson KA, Villemagne VL, Sperling RA, Alzheimer's Disease Neuroimaging Initiative, Australian Imaging, Biomarker and Lifestyle study of ageing, Harvard Aging Brain Study. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer's disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018 Sep;14(9):1193-1203. Epub 2018 May 24 PubMed.
- Greenfield A, Carrel L, Pennisi D, Philippe C, Quaderi N, Siggers P, Steiner K, Tam PP, Monaco AP, Willard HF, Koopman P. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet. 1998 Apr;7(4):737-42. PubMed.
- Miyake N, Mizuno S, Okamoto N, Ohashi H, Shiina M, Ogata K, Tsurusaki Y, Nakashima M, Saitsu H, Niikawa N, Matsumoto N. KDM6A point mutations cause Kabuki syndrome. Hum Mutat. 2013 Jan;34(1):108-10. Epub 2012 Oct 17 PubMed.
- Tang GB, Zeng YQ, Liu PP, Mi TW, Zhang SF, Dai SK, Tang QY, Yang L, Xu YJ, Yan HL, Du HZ, Teng ZQ, Zhou FQ, Liu CM. The Histone H3K27 Demethylase UTX Regulates Synaptic Plasticity and Cognitive Behaviors in Mice. Front Mol Neurosci. 2017;10:267. Epub 2017 Aug 24 PubMed.
- Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, Arnold AP, Voskuhl RR. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest. 2019 Aug 12;129(9):3852-3863. PubMed.
Further Reading
Papers
- Claus JJ, Van Gool WA, Teunisse S, Walstra GJ, Kwa VI, Hijdra A, Verbeeten B, Koelman JH, Bour LJ, Ongerboer de Visser BW. Predicting survival in patients with early Alzheimer's disease. Dement Geriatr Cogn Disord. 1998 Sep-Oct;9(5):284-93. PubMed.
- Heyman A, Wilkinson WE, Hurwitz BJ, Helms MJ, Haynes CS, Utley CM, Gwyther LP. Early-onset Alzheimer's disease: clinical predictors of institutionalization and death. Neurology. 1987 Jun;37(6):980-4. PubMed.
- Ueki A, Shinjo H, Shimode H, Nakajima T, Morita Y. Factors associated with mortality in patients with early-onset Alzheimer's disease: a five-year longitudinal study. Int J Geriatr Psychiatry. 2001 Aug;16(8):810-5. PubMed.
- Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, Marder K, Sano M, Devanand D, Albert SM, Bylsma F, Tsai WY. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997 Mar 12;277(10):806-12. PubMed.
- Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME, Vlassenko AG. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci U S A. 2019 Feb 4; PubMed.
Primary Papers
- Davis EJ, Broestl L, Abdulai-Saiku S, Worden K, Bonham LW, Miñones-Moyano E, Moreno AJ, Wang D, Chang K, Williams G, Garay BI, Lobach I, Devidze N, Kim D, Anderson-Bergman C, Yu GQ, White CC, Harris JA, Miller BL, Bennett DA, Arnold AP, De Jager PL, Palop JJ, Panning B, Yokoyama JS, Mucke L, Dubal DB. A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease. Sci Transl Med. 2020 Aug 26;12(558) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Mayo Clinic
For many diseases, sex differences profoundly affect the risk, underlying mechanisms for the development and progression of disease, symptoms, treatment response, and overall morbidity and mortality. Within the dementia field, especially Alzheimer’s disease dementia, there has been an increased interest in identifying sex differences on a number of fronts, but this research topic is still in its infancy. This manuscript by Davis et al., which is very well-written and methodologically sound, focuses and expands on two specific aspects of sex differences in Alzheimer’s disease (AD): mortality bias and distinguishing hormonal effects from sex (XY) chromosome effects.
First, the authors show, in a meta-analysis of cohort studies and among mice expressing a human amyloid precursor protein (hAPP), that male sex increased the risk for mortality, including among persons diagnosed with AD dementia. Giving that aging is the greatest risk factor for AD and other dementias, this study further highlights the importance of considering sex differences in mortality bias when conducting research on sex differences in risk factors and mechanisms for AD. Second, and perhaps even more intriguing, is their methodology to try to separate sex chromosomes from sex hormones. The authors uniquely show that the addition of an X (e.g., XX vs. XY) leads to brain resilience; thus, it is not the Y gene that is necessarily detrimental. This work suggests that the sex chromosomes largely governed the observed sex differences. However, the study did not specifically assess the activating effects of hormonal treatments, only loss of hormones through gonadectomy. Lastly, the authors demonstrate, through a number of experiments, that the gene Kdm6a, which escapes X inactivation, causes neural and cognitive resilience in the mouse studies. Thus, the more doses (i.e., XX vs. XY), the better resilience.
Notably, most GWAS studies have historically excluded the sex chromosomes. This study highlights the need to examine genes on the X and Y chromosomes for risk and resiliency to AD and other dementias. I look forward to the future results that will come from the focus on the sex chromosomes to better understand the sex differences for the risk and resiliency of AD.
Massachusetts General Hospital/Harvard Medical School
This study is a fascinating deep dive into the effects of the X chromosome itself on vulnerability/resilience factors associated with AD. Lots of interest has been focused on the dichotomous indicator of biological sex and its impact on risk in recent papers, however, they did not get into the mechanism behind which risk or resilience to AD might be sex-dimorphic. A fascinating fact about the XY and XX chromosome configuration is that the XX combination is a “double dose” of the X chromosome. In order to compensate for this issue, either the maternal or paternal X chromosome is randomly inactivated. Intriguingly, some genes on the inactivated X chromosome will escape this inactivation. This could be random, but could also be more systematic.
Kdm6a is a gene that more consistently escapes inactivation across the population, with a recent paper suggesting an increased risk for autoimmune diseases such as MS is due to its expression in CD4+ T lymphocytes in women (Itoh et al., 2020). The fact that increased Kdm6a gene expression was found to moderately associate with resilience to AD-related neurotoxicity and cognitive impairment in mouse models in the current paper alludes to the fact that downstream implications of this gene activation must be cell-type specific. This won’t be a one-size-fits-all risk gene.
An interesting point to consider is the parental origin of the X chromosome; while there is research building to now examine the implications of escaped inactivation of X chromosomes in association with AD risk, it could also be quite interesting to examine the impact of epigenetic diversity that can be imparted by either parental X depending on which is inactivated. Either way, this paper by Dr. Dubal’s lab is an exciting and novel examination of the mechanisms that underlie sex differences in AD risk/resilience.
References:
Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, Arnold AP, Voskuhl RR. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest. 2019 Aug 12;129(9):3852-3863. PubMed.
University of California, San Diego
Converging evidence demonstrates the enhanced reserve against Alzheimer’s disease that women show in comparison with men. However, it has been difficult to recapitulate this in animal models, which restricts the utility of translational research. Furthermore, the underlying mechanism for this sex difference remains unknown. Now, in this elegant series of experiments, Dr. Davis and her team led by Dr. Dubal manipulate various aspects of sex differences, including the presence or absence of gonads and second chromosomes, to hone in on the importance of a second X chromosome in protecting females from some of the deleterious effects of Alzheimer’s. They identify the candidate gene for a protective sex-linked effect, Kdm6a, which does not undergo X-linked inactivation. By crossing over into human tissue samples, they were able to identify the mRNA expression of KDM6A, finding enhanced expression in the temporal lobe but not the cerebellum across several datasets, especially in women.
Thus, this study taps into several exciting aspects of sex difference research in Alzheimer’s, including the importance of understanding genetic influences in men and women separately beyond apolipoprotein E, reflecting our own group’s recent findings (Fan et al., 2020). Furthermore, Davis and colleagues’ findings reflect a lack of sex difference in amyloid burden, consistent with findings from our own group and others’ that the sex difference in humans seems to be driven by excess tau deposition in women (Digma et al., 2020; Buckley et al., 2019). While sex differences in cognitive decline are perhaps more nuanced in humans, with women having an accelerated decline later in the MCI and in the early dementia period, and hence not showing sustained resilience, this paper is an important addition to our understanding of potential mechanisms of sex differences in Alzheimer’s.
References:
Fan CC, Banks SJ, Thompson WK, Chen CH, McEvoy LK, Tan CH, Kukull W, Bennett DA, Farrer LA, Mayeux R, Schellenberg GD, Andreassen OA, Desikan R, Dale AM. Sex-dependent autosomal effects on clinical progression of Alzheimer's disease. Brain. 2020 Jul 1;143(7):2272-2280. PubMed.
Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ, Alzheimer’s Disease Neuroimaging Initiative. Women can bear a bigger burden: ante- and post-mortem evidence for reserve in the face of tau. Brain Commun. 2020;2(1):fcaa025. Epub 2020 Apr 14 PubMed.
Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HI, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, Johnson KA, Sperling RA. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured By Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. 2019 Feb 4; PubMed.
Johns Hopkins University School of Medicine
Previous studies indicated that there were sex differences in mortality and cognitive deficits in human patients with Alzheimer’s disease (AD). This study by Davis et al. provides new evidence that sex chromosomes and their associated genes might play important roles in regulation of sex-specific vulnerability to AD.
Specifically, the study started with a meta-analysis of mortality data in human populations worldwide and found that in AD patients, males had about 62 percent increased risk for death compared to females. The same sex-difference results were obtained in a transgenic mouse model of AD, in which mutated human amyloid precursor protein (hAPP) was expressed. In addition to mortality, the study also used the hAPP transgenic mice to determine if there were sex differences in multiple cognitive functions, such as spatial learning and memory (Morris water maze) and passive-avoidance testing (fear memory). To eliminate the influences of sex hormones on mortality and cognitive functions, the study performed gonadectomy to deplete the circulating hormones. The results demonstrated that female mice performed significantly better in all tests, although the AD-related pathological features in the brain were similar between the two sexes. These results suggest that different sex chromosomes, but not sex hormones, might underlie the observed differences in vulnerabilities to AD.
To address the important roles of sex chromosomes, the study used two excellent mouse genetic models. One was the Four Core Genotype (FCG) mice, which allowed the generation of XX and XY mice, each with either female ovaries (XX-female, XY-female) or male testes (XX-male, XY-male). Together with gonadectomy, these mice allowed investigation of how sex chromosomes contribute to the disease of interest independent of sex hormones. By crossing these mice with hAPP mice, the study illustrated clearly that sex chromosomes determined the different vulnerabilities to AD between male and female mice. More specifically, the additional X chromosome in XX mice decreased the female vulnerability compared to XY mice. To further confirm the important roles of the X chromosome, a second set of transgenic mice were used, in which the Y* chromosome in the male mouse has a pseudoautosomal region recombining abnormally with the X chromosome during meiosis. As a result, crossing these XY* male mice with XX female mice generated XX and XO mice with ovaries and XY and XXY mice with testes. By crossing male XY* mice with female hAPP mice, the study revealed that XX-hAPP and XXY-hAPP mice survived longer than XY-hAPP and XO-hAPP mice, indicating one more X chromosome extended the life of AD mice. Similarly, the hAPP mice with two XX chromosomes had better cognitive function than those with only 1 X chromosome.
How does the additional X chromosome contribute to the reduced vulnerability of female mice to AD? The female XX mice only express one active X, with the other X inactivated. However, a small number of genes can escape such X inactivation and thus have higher expression in females. One such gene is Kdm6a (also known as Utx), which is the demethylase for H3K27. Indeed, the expression level of Kdm6a was always proportionally higher based on the number of X chromosomes. More importantly, the protein level of KDM6A is higher in the brains of women and genetic variation-induced KDM6A upregulation was associated with higher cognitive resilience to AD in humans. Functionally, knocking down Kdm6a in neurons of XX mice reduced their resilience to Aβ-induced neurotoxicity, whereas overexpression of Kdm6a in XY neurons enhanced their resilience. Surprisingly, in vivo overexpression of Kdm6a in adult XY-hAPP mice attenuated their vulnerability to AD-related cognitive impairments.
In addition to the main conclusion, there are several very interesting observations that warrant further investigation. First, the mice with only one X (XO mice) consistently showed higher mortality and cognitive deficits compared with the XY mice, suggesting that some genes in the Y chromosome might somehow play protective roles. Second, in human AD patients the expression level of Kdm6a was only higher in temporal cortex, a brain region affected in early AD, but not in cerebellum, a region often spared in AD. Such a region-specific effect probably indicates that Kdm6a upregulation is a natural response of neurons encountering neurodegenerative stimuli. Third, in the Y chromosome there is a Kdm6a paralog Uty, sharing high homology but without the histone demethylase activity. Therefore, although not tested directly, it is very likely that H3K27 methylation and the subsequent epigenetic regulation of gene transcription is involved.
The study did not explore further the cellular and molecular mechanisms by which X chromosome or Kdm6a regulated the observed neural responses to AD. For instance, by using the same transgenic mice, it will be of great interest to examine how adding one more X chromosome affects the neural development processes, such as neurogenesis, neuronal migration, axon growth and guidance, dendrite development, and synapse formation, either at the cellular or the circuit level. Advanced multiomics can be used to dissect out the differences in chromatin structure and transcriptomics of mature neurons. Are there other X chromosome genes besides Kdm6a that participate in the resilience of AD? Do non-neuronal cells in the nervous system participate in the sex-chromosomes-mediated effects?
Recent studies in both mouse and human neural stem cells demonstrated that Kdm6a knockout impaired dendritic development and synaptic plasticity during development and cognitive deficits in adult mice. One important future direction is to investigate how upregulation of Kdm6a in neurons, either during development or in adult animals, affects neuronal function at the cellular or molecular level. As a H3K27 demethylase, Kdm6a is certainly able to modulate the epigenetic landscape and the transcription of many downstream genes. Lastly, the fast-developing iPSC and organoid approaches would allow direct experiments with human neurons both at cellular level and the circuit level.
Collectively, this interesting study provides strong evidence that the X chromosome and the genes it harbors regulate the sex-based differences in symptoms of AD patients. In particular, Kdm6a overexpression in adult mice enhanced cognitive function in male mice, suggesting a potential therapeutic treatment for AD. More importantly, the research strategy and the genetic mouse models can be used to explore if sex chromosomes can regulate other neurodegenerative diseases, or more generally neurological diseases that show sex-based differences in symptoms.
University of California, San Francisco
This is a provocative and exciting study that addresses the sex-specific effects in Alzheimer’s disease, an understudied topic with important impact on both basic mechanistic and translational efforts. Here, the dissociation of the sex chromosomes and sex hormones is rigorously investigated. The role of X chromosome-dosage effects, especially the effects of Kdm6a, is compelling.
The study focuses on amyloid-mediated cognitive deficits. Sex-specific effects are also observed in response to tau pathology in mice, with male microglia exhibiting more transcriptomic changes than female microglia (Kodama et al., 2019).
The resilience observed in females could also potentially be modified by genetic risk alleles. For example, female ApoE4 knock-in mice appear to be more impaired cognitively than males. R47H mutation of TREM2 allele also appears to induce more severe cognitive decline in female mice than males, especially in the presence of tau pathology (Sayed et al., 2020).
References:
Kodama L, Guzman E, Etchegaray JI, Li Y, Sayed FA, Zhou L, Zhou Y, Zhan L, Le D, Udeochu JC, Clelland CD, Cheng Z, Yu G, Li Q, Kosik KS, Gan L. Microglial microRNAs mediate sex-specific responses to tau pathology. Nat Neurosci. 2020 Feb;23(2):167-171. Epub 2019 Dec 23 PubMed.
University of Wisconsin-Madison
I have to applaud the researchers' use of intriguing methods to try to separate the effects of sex hormones from the genetic effects of sex chromosomes. Based on their report, it looks like they've found ways to address some of the primary questions that typically come up in many of the sex-specific studies of AD; namely whether sex differences are due to hormonal differences, genetic differences in the sex chromosomes, or other differences, such as environment.
On the surface it looks like they've managed to control for potential confounding by hormonal and sex chromosome differences by removing one in the presence of the other (i.e., the XY mice with testes or ovaries and the XX mice with testes or ovaries), but I don't have enough experience in sexual biology to say what other complications could occur with these manipulations that could influence the results. As the authors pointed out, in addition to the usual limitations of modeling AD in mice, the study did not include other sex chromosome-based biological functions and they only focused on Kdm6a. Nevertheless, this paper is very intriguing and raises important questions and exciting avenues for exploration in human research.
Make a Comment
To make a comment you must login or register.