Does Tangle Progression Follow ApoE Expression?
Quick Links
The spread of tau tangles through the brain in Alzheimer’s disease is anything but random, and researchers long ago charted a stereotypical path that is now known as Braak staging. But what factors steer this march of pathology? According to a study published July 27 in Science Translational Medicine, regional expression of ApoE, SLC1A2, and a cadre of other genes may dictate when and where neurofibrillary tangles emerge.
- A tau-PET algorithm charted the spread of tau aggregates through the brain.
- Expression of 577 genes correlated with regional vulnerability.
- ApoE and synaptic proteins correlated most strongly.
Superimposing tau-PET maps with high-resolution spatial transcriptomics, scientists led by Jorge Sepulcre at Massachusetts General Hospital in Boston related expression of nearly 600 genes to tangle spread throughout the brain. Their findings suggest that gene expression dictates regional vulnerability to tau aggregation, but does not distinguish mechanisms driving the apparent spread, such as neuron-to-neuron propagation versus local aggregation.
The study takes a fresh approach to tracing spread of tangles throughout the brain. While previous work had superimposed tangle distribution onto standardized maps of structural or functional connectivity, in this study first author Victor Montal and colleagues let go of the predetermined brain connectivity maps. Instead, they simply asked how tau tangles were distributed throughout the brain by developing a tau-connectivity network based solely on relative tangle accumulation from one region to another. This unbiased approach allowed them to remain agnostic about how tau pathology might spread, Montal said.
To piece together a network, Montal and colleagues looked at tau-PET scans from cognitively normal volunteers who had accumulated Aβ plaques. Nineteen were from the Harvard Aging Brain Study (HABS); 52 came from the AD Neuroimaging Initiative (ADNI). By comparing levels of tau accumulation voxel by voxel across the cortex, the researchers found 58 “supernodes.” Each supernode comprised several regions with similar levels of tau accumulation. They were found across the whole spectrum of Braak staging, with those in later Braak regions having fewer tangles than supernodes in earlier-stage regions.
Next, the researchers applied an algorithm based on graph theory to develop a network connecting these various nodes. This network predicted the longitudinal progression of tangle pathology from one region to another. The scientists validated this progression on a subset of volunteers who had undergone follow-up scans. The upshot? A person’s tau-PET-based network accurately predicted which regions would accumulate tangles from one scan to the next, in other words, which were most vulnerable.
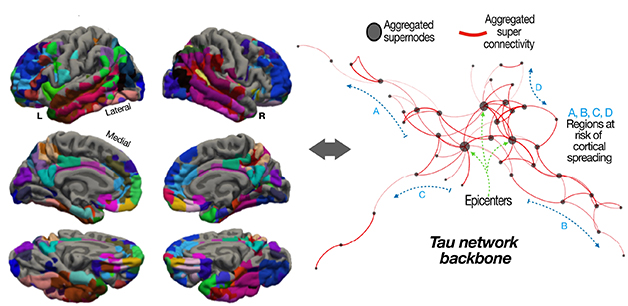
Network of Entanglement. Relationships between levels of tau tangles in 58 supernodes (designated by different colors, left), predicted the spread of this pathology across the brain (right). [Courtesy of Montal et al., Science Translational Medicine, 2022.]
What factors dictated a brain area’s vulnerability to being invaded by tangles? Montal addressed this question using high-resolution transcriptomic data from the Allen Human Brain Atlas, which comprises bulk RNA-Seq from regional samples across the brain. Essentially, the scientists compared gene-expression patterns in regions within each tau network supernode, and looked for genes whose regional expression patterns matched that region’s vulnerability. They identified 744 genes whose regional expression pattern tracked with vulnerability. Among those, 577 were found in postmortem brain samples from the ROS-MAP cohort, which includes people who had died in various stages of AD, so the authors focused on those.
What were the nuggets of this transcriptomic trove? For one, it was replete with genes involved in neuron structure and synaptic function. In particular, SLC1A2, which encodes a protein responsible for whisking glutamate out of the synaptic cleft, was strongly tied to vulnerability to tangles. Its apparent role in spreading meshes with the idea that excitatory neurons are most vulnerable to tau accumulation.
ApoE also prominently featured. The greater its expression in a given region, the more susceptible that region was to neurofibrillary tangles.
ApoE is primarily an astrocytic gene. How might it render a region prone to tau aggregation? This is unclear, but the finding jibes with previous work. For example, a recent study led by Jason Ulrich and David Holtzman at Washington University in St. Louis reported that nixing ApoE expression from astrocytes lessened tau pathology, and neurodegeneration, in transgenic mice (Apr 2021 news). Other studies have spotted ApoE mingling within neurofibrillary tangles (Drummond et al., 2020).
“[The studies] raise the question of how and where a secreted, glial-expressed protein like APOE could associate with a predominantly intracellular, neuronal protein like tau,” Ulrich commented. “This could happen in the extracellular space or perhaps in autophagic-lysosomal compartments in neurons or glial cells.”
Another finding supporting ties between ApoE and tau pathology comes from carriers of the protective Christchurch variant of ApoE3. In a woman who also carried an autosomal-dominant AD mutation, the protective ApoE variant fended off tau pathology despite massive amyloid accumulation (Nov 2019 news). —Jessica Shugart
References
News Citations
- Squelching ApoE in Astrocytes of Tau-Ravaged Mice Dampens Degeneration
- Can an ApoE Mutation Halt Alzheimer’s Disease?
Paper Citations
- Drummond E, Pires G, MacMurray C, Askenazi M, Nayak S, Bourdon M, Safar J, Ueberheide B, Wisniewski T. Phosphorylated tau interactome in the human Alzheimer's disease brain. Brain. 2020 Sep 1;143(9):2803-2817. PubMed.
Further Reading
Primary Papers
- Montal V, Diez I, Kim CM, Orwig W, Bueichekú E, Gutiérrez-Zúñiga R, Bejanin A, Pegueroles J, Dols-Icardo O, Vannini P, El-Fakhri G, Johnson KA, Sperling RA, Fortea J, Sepulcre J. Network Tau spreading is vulnerable to the expression gradients of APOE and glutamatergic-related genes. Sci Transl Med. 2022 Jul 27;14(655):eabn7273. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University
It is very interesting that APOE expression came up in this study as being associated with the spread of tau pathology. This observation tracks with some recent studies from our group that found reducing APOE levels through antisense oligonucleotides or removal of astrocytic APOE expression reduced tau pathology and tau-dependent neurodegeneration in the P301S tau model.
The study also brought to mind a very nice recent paper from Oskar Hansson’s group (Vogel et al., 2021) that described subtypes of tau, one of which, a medial-temporal lobe-sparing form, showed a bias toward APOE4 allele noncarriers. Understanding how APOE genotype, or other genetic factors such as TREM2, influence tau propagation networks will add another layer of nuance.
The mechanism by which APOE would influence NFT burden is still unclear. As the authors note, interactome studies of NFTs and p-tau protein also identified APOE, raising the question of how and where a secreted, glial-expressed protein like APOE could associate with a predominantly intracellular, neuronal protein like tau. This could happen in the extracellular space or perhaps in autophagic-lysosomal compartments in neurons or glial cells.
References:
Vogel JW, Young AL, Oxtoby NP, Smith R, Ossenkoppele R, Strandberg OT, La Joie R, Aksman LM, Grothe MJ, Iturria-Medina Y, Alzheimer’s Disease Neuroimaging Initiative, Pontecorvo MJ, Devous MD, Rabinovici GD, Alexander DC, Lyoo CH, Evans AC, Hansson O. Four distinct trajectories of tau deposition identified in Alzheimer's disease. Nat Med. 2021 May;27(5):871-881. Epub 2021 Apr 29 PubMed.
Make a Comment
To make a comment you must login or register.