The Epidemic in Your Head? New Model Casts Amyloid as Intra-Brain Contagion
Quick Links
Whether via forest paths that link villages, buses that connect cities, or planes that cross continents, contagious diseases turn into epidemics by exploiting frequent travel corridors. A new study suggests that neurodegenerative disease spreads the same way—at least within the brain. Led by Alan Evans at the Montreal Neuroimaging Institute in Quebec, researchers created an outbreak-based mathematical model to describe the spread of misfolded proteins throughout the brain in neurodegenerative disease. They used the massive imaging dataset from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to optimize their model. It affirmed that, like infectious disease, Aβ spreads from one region of the brain to another via well-traveled routes. Published November 20 in PLOS Computational Biology, the model also predicted that clinical progression depends more on how fast the brain rids itself of Aβ than on how much Aβ it produces.
Researchers have come to realize that neurodegenerative diseases spread through networks, as regions of the brain connected via vast white-matter tract superhighways tend to accumulate disease in a synchronized fashion (e.g., Palop et al., 2006, and Apr 2009 news story). Animal studies suggest that Aβ and other pathogenic proteins may use these routes to spread from one cell to another across the brain (e.g., Jucker et al., 2013).
Constructing standard models that explain the spread of disease based on observational studies alone has proven difficult, so researchers are turning to mathematical modeling to gain a broader understanding of disease progression. “The development of most therapies is based on observational data,” said first author Yasser Iturria-Medina. “But there is not enough data driving analysis, and not enough variables considered.”
In 2012, a study led by Michael Weiner of the University of California, San Francisco, developed the so-called Network Diffusion Model, which predicted that pathogenic proteins spread throughout the brain along neural networks via diffusion. They relied on observations of gray-matter atrophy patterns in people with AD and frontotemporal dementia to validate their model (see Raj et al., 2012).
Inspired by this model and seeking to add in more parameters, Evans and colleagues developed the Epidemic Spreading Model to characterize the movement of proteins throughout the brain. The core premise was that the probability of a given brain region’s “infection” would rely on its degree of connectedness with affected areas. Unlike the Network Diffusion Model, the Epidemic Spreading Model would also account for the brain’s ability to clear pathogenic proteins.
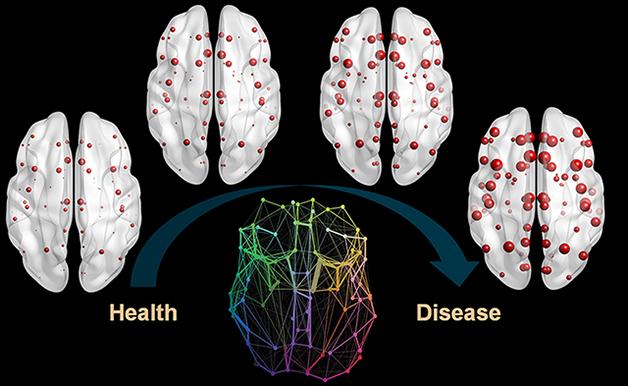
Brain Outbreak. Misfolded Aβ spreads throughout the brain via its structural connectome (bottom center), eventually accumulating (red) at various pit stops along the way as the disease progresses (left to right). [Image courtesy of Iturria-Medina et al., PLOS Computational Biology, 2014.]
Iturria-Medina and colleagues used two complementary data sets to anchor and test their model. They drew upon data from diffusion MRI taken on 60 young, healthy volunteers to develop an atlas of white-matter tracts, i.e., potential routes of transmission. They then looked to the ADNI imaging dataset—including amyloid PET scans of 733 people—to see if their model could predict bona fide pathogenic protein spread. Volunteers included 193 healthy controls, 233 people with early mild cognitive impairment (MCI), 196 with late MCI, and 111 with probable AD.
For each person in the cohort, the researchers created a map of Aβ deposition across 78 different regions in the brain. To pinpoint the source of the outbreak, the researchers plugged in several combinations of potential brain regions, ran the model, and compared the results to the ADNI PET imaging data. The model came closest to predicting actual Aβ deposition patterns when the cingulate cortex was used as the “outbreak epicenter,” or the source of Aβ gone wrong. With the cingulate as the seed, the researchers found their model could explain roughly half of the variance in Aβ deposition patterns between members in the cohort. They found that Aβ spread most readily to regions connected via white-matter tracts, and not necessarily those that were closest as the crow flies. Regions most connected to the epicenter developed Aβ deposits first, and regions with the most connections, also known as network hubs, developed the heaviest load.
The researchers used their model to estimate age at onset of Aβ “infection,” rate of Aβ production, and rate of Aβ clearance for each volunteer, and then looked for relationships between these factors and the spread of the disease throughout the brain.
A standout finding from the study was that a slowed rate of Aβ clearance correlated with a more advanced clinical diagnosis more strongly than did heightened Aβ production or younger onset age. Clearance rates also dropped in proportion to the number of ApoE4 alleles a person harbored. Presence of the risk allele also correlated strongly with a younger onset age, and less so with higher Aβ production rates.
The researchers incorporated CSF concentrations of Aβ1-42, total tau, and phospho-tau into their model, and compared them with the other disease characteristics. As expected, CSF Aβ1-42 concentration was strongly affected by rates of Aβ production and clearance. Intriguingly, so were CSF p-tau and t-tau, suggesting a relationship between Aβ pathology and tau. However, tau levels were even more strongly correlated with Aβ onset age and chronological age. According to the authors, these findings suggest that the duration of the relationship between tau and Aβ pathology is most important in dictating changes in tau.
Iturria-Medina ran Weiner’s Network Diffusion Model on the ADNI dataset, and found that while both models pointed to the cingulate as the source of Aβ spread, the Epidemic Spreading Model more accurately predicted the subsequent distribution of Aβ pathology throughout the brain. However, both models achieved a similar goal, commented Ashish Raj of Weill Cornell Medical College in New York, the first author of the Network Diffusion Model study. “Progression is stereotyped once you have a model that can capture how it spreads,” Raj said. “These researchers do this quite well with a sophisticated model that achieves this purpose.” Both Raj and Iturria-Medina said that the model should prove useful to study other nomadic pathogenic proteins as well, such as tau and α-synuclein.
One new finding to come out of Iturria-Medina’s model was the importance of Aβ clearance on disease progression. This may support the idea that drugs should be aimed at boosting clearance, rather than dousing production, Raj said.—Jessica Shugart
References
News Citations
Paper Citations
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006 Oct 19;443(7113):768-73. PubMed.
- Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013 Sep 5;501(7465):45-51. PubMed.
- Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012 Mar 22;73(6):1204-15. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Evans AC, Alzheimer's Disease Neuroimaging Initiative. Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders. PLoS Comput Biol. 2014 Nov;10(11):e1003956. Epub 2014 Nov 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.