FDA Approves Amyvid for Clinical Use
Quick Links
The Food and Drug Administration (FDA) has approved Amyvid (florbetapir F18 injection), Avid Pharmaceuticals/Eli Lilly and Company's positron emission tomography (PET) tracer, for Aβ plaque imaging in cognitively impaired patients being clinically evaluated for Alzheimer’s disease. The compound will be available for use in June, with the aim of helping researchers rule out—not diagnose—the disease.
"Amyvid is the first and only FDA-approved radioactive diagnostic agent for brain imaging of amyloid plaques in patients," said Daniel Skovronsky of Avid in a telephone press briefing today. Approval "offers physicians a tool that, in conjunction with other diagnostic evaluation, can provide information to help physicians evaluate their patients."
Amyvid's main purpose will be to rule out AD; a negative scan means little or no plaque is present, which effectively rules out AD as the cause of the person’s cognitive decline. This could help the approximately one in five patients who are diagnosed with Alzheimer's but later at autopsy show no evidence of the disease. "A physician can further evaluate [a patient] to try and figure out what they really have," Skovronsky told Alzforum. However, since plaques can be present in both people with or without AD, a positive scan—indicative of moderate to frequent plaques—would not establish an Alzheimer's diagnosis. Further, Amyvid is not approved to monitor plaque response to therapies or predict risk for dementia, Skovronsky said.
An FDA advisory panel temporarily rejected Avid Pharmaceutical's new drug application in January of 2011, pending better inter-reader reliability of the scans (see ARF related news story). In answer to the specific requests the FDA issued in March 2011, the company has since developed a binary method of reading the scans, as well as a training program to teach radiologists and nuclear medicine physicians how to evaluate them. In place of the previous five-point, semi-quantitative method, black-and-white scans are now read as positive or negative, based on the radioactive contrast between cerebral cortex gray matter and adjacent white matter (the cerebellum radioactivity does not come into play). For instance, a negative scan shows more radioactivity in white matter than gray, creating a distinct border between the two. Positive scans lack that clear definition in two or more brain areas, making normally distinct borders appear blurred, or gray matter radioactivity may even surpass that of nearby white matter (see picture below). Noisy scans, those of significantly atrophied brains, and scans of patients who move during the procedure may be more difficult to interpret. (For more information, see Amyvid's full prescribing information.)
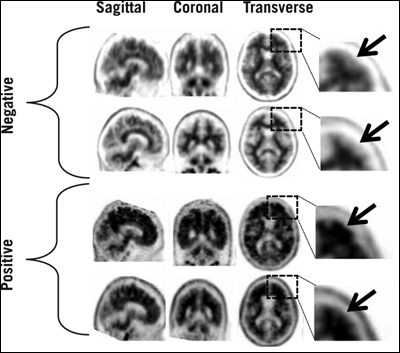
In negative scans, gray matter shows less cortical radioactivity than does the adjacent white matter, preserving clear borders between the two areas. But in positive scans, gray-white contrast drops, and cortical radioactivity is more similar—or even exceeds—that of the nearby white matter. Image credit: Eli Lilly and Company
A three-hour training program for radiologists and nuclear medicine physicians will be available both online (a link to the program will appear later on the Amyvid website) and in person to teach readers how to interpret the scans (see ARF related news story). In previous trials of the training program, in-person training resulted in 96 percent sensitivity and 100 percent specificity in reading scans taken within a year of a patient's death. For people trained remotely online, sensitivity averaged 82 percent and specificity 95 percent.
PET scans using Amyvid will not be covered by Medicare at the time of the June launch, said a Lilly representative. The company, with industry and trade associations as well as professional societies, is lobbying to change that. Partnering with the American College of Radiology and the Society of Nuclear Medicine, Lilly has requested formal reconsideration of the non-coverage policy by the Center for Medicare and Medicaid Services, a process that could take nine months or more, said the representative. Amyvid's cost may limit its initial use; each dose will cost roughly $1,600, though that will vary depending on private insurance coverage and where the scan is conducted.
"The approval of PET amyloid imaging for clinical use is definitely an important step
forward for the field," Reisa Sperling, Brigham and Women's Hospital, Boston, Massachusetts, told Alzforum in an e-mail (see full comment below). "PET amyloid imaging will prove useful in the clinic, especially for those difficult dementia cases—younger patients, unusual clinical presentations, or apparently rapidly progressing dementias," she added.
But clinicians have their work cut out for them in terms of developing careful guidelines and recommendations for how Amyvid and similar compounds are used, said Michael Weiner, University of California, San Francisco. "Hopefully there will not be a lot of abuse," he told Alzforum. "What we don't want to see is some for-profit imaging center starting to advertise 'Get your test for Alzheimer's disease here,' and making a lot of money off of it. That's not appropriate." It will be essential for a PET scan with Amyvid to be responsibly put in the context of additional neurological, psychological, and physical tests, as well as family history, to make an accurate diagnosis, he said.
In addition, more data will be needed to clarify the scan's credibility, said William Jagust, University of California, Berkeley. "We don't know what the false-negative and false-positive rates for this test are going to be," he told Alzforum, adding "It's not absolutely certain that a negative scan can rule out the presence of Alzheimer's disease." Jagust, too, emphasized the need to take all other clinical data into consideration when making a diagnosis, and not using a PET scan with Amyvid as a standalone diagnostic.
Skovronsky agreed, and looked toward implications for the future. "Hopefully now we are entering a new age, where we can evaluate patients not based on their symptoms, but based on the molecular pathology in their brain," he said. "We hope that will lead not just to improvements in evaluating patients, but to the generation of therapies that don't target symptoms, but target the molecular pathology."—Gwyneth Dickey Zakaib.
References
News Citations
- Committee Shoots Down Florbetapir, Raising Bar for Field at Large
- Miami: Can the Naked Eye Tell When a Scan Is Positive?
External Citations
Further Reading
Papers
- Hsiao IT, Huang CC, Hsieh CJ, Hsu WC, Wey SP, Yen TC, Kung MP, Lin KJ. Correlation of early-phase (18)F-florbetapir (AV-45/Amyvid) PET images to FDG images: preliminary studies. Eur J Nucl Med Mol Imaging. 2012 Apr;39(4):613-20. PubMed.
News
- Phase 3 Paper on Amyloid Ligand Out Just Before FDA Meeting
- Miami: Amyloid PET in the Clinic: What Are the Issues?
- Miami: Scan and Tell? Amyloid Imaging Confronts Disclosure Dilemma
- Love a Good Fight? Check Out Brouhaha Over Amyloid Imaging
- Committee Shoots Down Florbetapir, Raising Bar for Field at Large
- Miami: Can the Naked Eye Tell When a Scan Is Positive?
Annotate
To make an annotation you must Login or Register.

Comments
Mayo Clinic College of Medicine
I am excited that an amyloid imaging agent will now be available
because there are instances where my patients, their families, and I
would appreciate the specific information about Alzheimer's disease
that it would provide. My hope is that ordering and interpretation of
amyloid imaging will be limited to physicians skilled in the diagnosis
of dementia. I am worried about the many possibilities for misuse of
the test. Even if a nuclear medicine physician has taken the course
provided by Lilly, any physician who has low instances of contact with
the test is likely to make mistakes in interpretation.
Risks for overdiagnosis of Alzheimer's are worrisome, especially when
the amyloid imaging is not interpreted in light of the clinical history.
For example, up to 30 percent of cognitively normal people over age 70
years are expected to have a "positive" scan. As most physicians and
most lay people do not understand the distinction between "Alzheimer's disease" and "dementia," hearing the former is likely to be misinterpreted as
the latter.
Center for Alzheimer Research and Treatment, Brigham and Women's Hospital, Massachusetts General Hospital, Harvard Medical School
The approval of PET amyloid imaging for clinical use is definitely an
important step forward for the field. I think PET amyloid imaging will
prove useful in the clinic, especially for those difficult dementia cases—younger patients, unusual clinical presentations, or apparently rapid
progressing dementias. Probably the most helpful information right now
will be in those cases where the scan is negative, as the evidence
suggests that a negative amyloid scan makes the diagnosis of Alzheimer's
disease dementia much less likely and other causes of dementia should be
investigated.
We need more research to understand the positive predictive
value of PET amyloid imaging, particularly as we move earlier in the
disease process into early mild cognitive impairment. I do not think we
should use amyloid imaging as a screening tool in clinically normal
individuals at this point, as we don't have enough data to quantify the
increase in risk or the timeframe for progression towards AD dementia, but
hopefully that research will continue to help us understand the
preclinical stages of AD.
IUPUI
There is a lot of excitement now that Eli Lilly announced that the U.S. Food and Drug Administration had approved Amyvid for use as a diagnostic modality for Alzheimer’s disease (AD). This certainly is a tremendous advance for the Alzheimer’s field. There is no doubt that neurologists and other specialists trained in dementia evaluation will greatly benefit from utilizing Amyvid to distinguish AD from other causes of dementia. Dementia specialists agree that good clinical practice mandates that they add this test to their routine dementia workup strategy and not substitute the standard dementia workup with Amyvid scans. (A routine dementia workup typically consists of detailed neurological and cognitive evaluation, a structural scan of the brain for evaluation of reversible causes for cognitive decline and vascular changes, and targeted laboratory workup for other potential causes for cognitive decline.)
It seems that Amyvid scans will mostly aid physicians in diagnosing or ruling out dementia of the Alzheimer’s type, especially in complex and challenging cases of dementia and in mild cognitive impairment (MCI), where a positive scan could implicate AD as the cause of cognitive changes. Still, most experts agree that cognitively normal persons should not be subjected to this type of diagnostic imaging. A third to a half of all cognitively normal persons above the age of 65 years would have a positive amyloid scan. It remains unclear (and an area of intense study) what the implications of a positive scan are in cognitively normal persons. Until this is better understood, these scans should not be offered to those who have no cognitive symptoms.
Make a Comment
To make a comment you must login or register.