Higher-Resolution Spatial Transcriptomics Maps Mayhem Near Plaques
Quick Links
Good news for the curious scientist: Spatial transcriptomics got another bump in resolution. In Nature Neuroscience on February 2, researchers led by Morgan Sheng and Xiao Wang of the Broad Institute at Massachusetts Institute of Technology introduce STARmap PLUS, a technique to track the transcriptomes of individual cells in situ while simultaneously detecting adjacent proteins. Its ability to measure transcripts from more than 2,000 genes within tiny 100 nm cubed tissue samples represents a significant improvement on previous methods.
- A new method marries spatial transcriptomics with protein detection in the same sample.
- With a 100 nm voxel size, spatial resolution surpasses that of previous methods.
- In AD mouse model, it maps single-cell gene expression to histopathology.
STARmap PLUS allowed the scientists to compare gene expression of individual cells at slightly different distances from Aβ plaques in a mouse model of amyloidosis. They found that microglia on the front lines—within 10 microns of a plaque—expressed a cadre of disease-associated genes, while their counterparts stationed just a few microns away held a more homeostatic composure. Activated astrocytes also encircled plaques, but lingered 10 to 20 microns away from the front lines. Neurons were relatively scarce in this zone and, in the few that remained, synaptic function was downregulated.
“This beautiful data nicely corroborated previous identifications of disease-associated microglia and astrocytes, and comprehensively provided a spatial relationship of these disease-associated populations with plaque and tau pathologies,” commented Yingyue Zhou and Marco Colonna of Washington University in St. Louis. “The results showed that the STARmap PLUS technology can be widely used to study changes in gene signatures in 4D, combining space and time, through disease progression.”
“It is clear that with this type of analysis, the field is transitioning in a new phase of understanding of the pathogenesis of Alzheimer’s disease,” wrote Bart De Strooper of KU Leuven in Belgium. “The emerging picture is that the amyloid deposits in the brains of mice and humans are not innocent. They induce a mainly microglia-driven multicellular response, most similar to a chronic inflammation.”
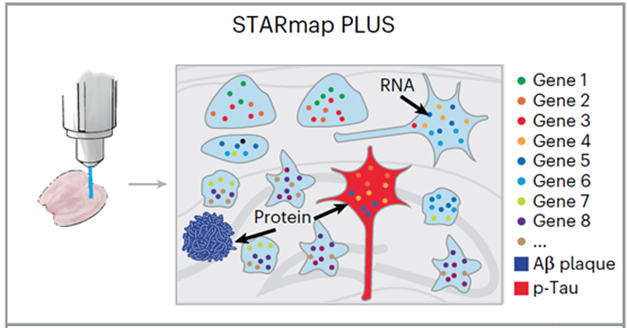
The Next Frontier? At a resolution of below 100nm, STARmap PLUS simultaneously measures more than 2,000 transcripts, plaques, and p-tau. [Courtesy of Zeng et al., Nature Neuroscience, 2023.]
The study is the latest advance within the burgeoning field of spatial transcriptomics. Researchers have developed several approaches to map the transcriptomes of cells within the brain. Using chip-based methods—in which brain slices are blotted with chips dotted with nucleic acid probes—researchers led by De Strooper used spatial information from adjacent brain slices to map plaques in one slice, and transcriptomes of single cells in another. This allowed them to discern plaque-induced genes, aka PIGs, that cells expressed in close proximity to Aβ deposits (Aug 2019 news; Jul 2020 news). Other researchers have boosted the low resolution of chip-based spatial transcriptomics with computational algorithms (Aug 2022 conference news). Even so, resolution, and inability to decipher transcriptomes in three-dimensional space, remain major limitations of such chip-based methods. They are also unable to simultaneously detect proteins—such as those in amyloid plaques and tau tangles—along with transcriptomes within a given brain slice.
To overcome these hurdles, Wang, then a postdoc in Karl Deisseroth’s lab at Stanford University, developed a technique called spatially resolved transcript amplicon readout mapping (STARmap, Wang et al., 2018). Its first step involves specialized pairs of probes to bind and then amplify a targeted set of mRNA transcripts within fixed brain tissue. As such, the method is biased toward transcripts of choice. After amplifying the bound transcripts in situ, the brain section is polymerized into a hydrogel, a process that both locks amplified transcripts in place and clears the tissue for better visibility. Using fluorescently labeled nucleic acid barcodes specific for each chosen transcript, Wang and colleagues then quantified each in three-dimensional space. They did so at subcellular resolution, i.e., within a miniscule voxel size of just 95x95x350 nm.
In the new paper, Wang, who now runs her own lab at the Broad, refined the method by adding a protein detection step into the workflow. It identified hyperphosphorylated tau using the AT8 antibody, and amyloid plaques with the dye X-34. The scientists also upped the number of transcripts they could detect, from 1,024 in the initial STARmap to more than 2,000 in STARmap PLUS.
To test what STARmap PLUS is capable of, co-first authors Hu Zeng, Jiahao Huang, Haowen Zhou, and colleagues tried it on a well-characterized TauPS2APP model, which overexpresses mutant forms of human APP, presenilin 2, and tau. When these mice reach 4.5 months of age, Aβ plaques emerge, then grow exponentially between 6 and 9 months, at which point neurons become damaged. Hyperphosphorylated tau accumulates within their neuronal cell bodies and axon bundles. The researchers collected brain slices from the hippocampi and three cortical brain regions of 8- and 13-month-old TauPS2APP and control mice, and used STARmap PLUS to measure transcripts from a curated list of 2,766 genes. “We used dye to study plaques, but in the future we could add different antibodies that target soluble Aβ and their oligomers, which may already be present at earlier timepoints,” Wang told Alzforum.
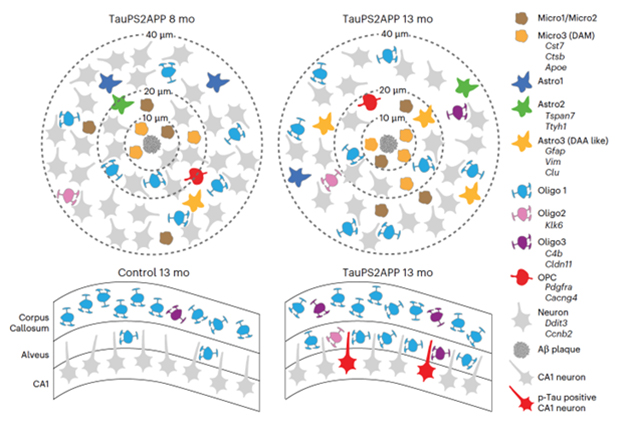
What findings stood out from this analysis? The greater spatial resolution of STARmap paid off around Aβ plaques, where it distinguished cell types at subtly different distances from them (image below). Within 10 microns of a plaque, microglia were the most abundant cell type by far; what's more, they appeared to interact directly with the plaque surface. Microglia were also concentrated in the 10-30 micron annulus, but there, other cells, including astrocytes, oligodendrocytes, and oligodendrocyte progenitor cells joined the crowd. Neurons of all subtypes were scarce within 10 microns of plaques.
The scientists also measured the intensity of hyperphosphorylated tau within 20 micron blocks throughout the tissue sample. In regions with higher p-tau, only oligodendrocytes were enriched of all cell types examined.

Bedazzled Brain. STARmap PLUS mapped the transcriptomes of 15 cell types (color legend, bottom right) in the cortices and hippocampi of TauPS2APP mice. It also showed how close a cell was to plaques (black, right top) and p-tau (pink, middle right). [Courtesy of Zeng et al., Nature Neuroscience, 2023.]
More than for any other cell type examined, microglial gene expression was driven by proximity to a plaque. The scientists identified three clusters of microglia based on the suite of genes they expressed. Micro1 and Micro2 held distinct homeostatic gene-expression signatures, while Micro3 closely aligned with a previously reported disease associated microglia (DAM) signature (Jun 2017 news). The proportion of microglia in the latter state increased near plaques, such that 70 percent of the cells within 10 microns of plaque were DAMs (image below). Homeostatic microglia were found in both control and TauPS2APP mice; in contrast, the DAM-like cells were only in the transgenic mice, and from 8 to 13 months they grew in number. In toto, the researchers proposed a local state transition from Micro1 to Micro2 to Micro3 near plaques during disease progression.

Plaque Mob. Microglial density shoots up close to plaque (left, x axis in microns). Most assume a DAM-like (orange) rather than a homeostatic state (green, blue). Right shows example of microglia (orange, green) surrounding plaques (black), with p-tau clustered nearby (pink). [Courtesy of Zeng et al., Nature Neuroscience, 2023.]
Astrocyte gene expression also changed with proximity to plaques, though less dramatically. As with microglia, three transcriptional clusters predominated—two with a homeostatic bent and one that corresponded to a previously reported disease-associated astrocyte (DAA) signature (Habib et al., 2020). Unlike with microglia, all three states were detected in control as well as TauPS2APP mice, although the proportion of DAA was higher in transgenics and rose from 8 to 13 months. In 8-month-old TauPS2APP mice, both homeostatic and DAA astrocytes were milling at a 10 to 40 micron distance from plaques. By 13 months, astrocytes were predominantly in the DAA state, suggesting they may be influenced by the microglial response near plaques.
The scientists also spotted spatial relationships between transcriptomes and pathology in cells of the oligodendrocyte lineage. They detected four transcriptional clusters, including three mature clusters—dubbed Oligo1, 2, 3—and one progenitor cell (OPC) cluster. All four massed within 10 to 40 microns of plaques at both 8 and 13 months. Curiously, while OPCs mostly gathered at 10 to 20 microns from plaques, mature oligodendrocytes tended to abound slightly farther afield, at 20 to 40 microns, with Oligo1 cells representing the lion’s share.
All four oligodendrocyte subtypes were also prominent in areas that contained high levels of p-tau, such as the CA1 region of the hippocampus, even in the absence of plaques. Interestingly, neurons were scarce near plaques, and those that did linger close by had higher levels of p-tau. The authors suspect oligodendrocytes could be attempting to repair dystrophic neurites in these plaque-adjacent, p-tau+ neurons.
Finally, the researchers integrated their analyses across cell types, looking at genes that changed expression based on nearness to plaques, aka spatially differentially expressed genes. It came as no surprise that these so-called SDEGs nearest to plaques were microglial, and part of the DAM signature. These microglial SDEGs partially overlapped with De Strooper’s previously reported PIGs (Chen et al., 2020). At an intermediate distance from plaques, SDEGs hailed from microglia, astrocytes, and oligodendrocytes (image below).
Core Versus Shell. STARmap PLUS suggests microglia form the initial cellular response to plaques early in AD, while other glial cells subsequently form an outer ring. Oligodendrocytes gravitate to p-tau+ neurons (bottom). [Courtesy of Zeng et al., Nature Neuroscience, 2023.]
All data considered, the scientists proposed a core-shell model of glial cells surrounding amyloid plaques, “where DAM emerge early in disease near plaques as the core, and the shell is a gliogenesis zone enriched for DAA-like cells, OPC, and oligodendrocytes that develop at a later stage, perhaps dependent on the formation of the inner ring of reactive microglia.”
Next, Wang plans to take STARmap PLUS into the AD brain (or rather, samples taken from it). She will also investigate how glia near plaques respond to anti-amyloid antibodies.—Jessica Shugart
References
News Citations
- Spatial Transcriptomics Uncovers Coordinated Cell Responses to Amyloid
- Paper Alert: Those PIGs! Spatial Transcriptomics Add Human Data
- High-Res Spatial Transcriptomics Offers New Views of Mouse Brain
- Hot DAM: Specific Microglia Engulf Plaques
Research Models Citations
Paper Citations
- Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J, Nolan GP, Bava FA, Deisseroth K. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science. 2018 Jul 27;361(6400) Epub 2018 Jun 21 PubMed.
- Habib N, McCabe C, Medina S, Varshavsky M, Kitsberg D, Dvir-Szternfeld R, Green G, Dionne D, Nguyen L, Marshall JL, Chen F, Zhang F, Kaplan T, Regev A, Schwartz M. Disease-associated astrocytes in Alzheimer's disease and aging. Nat Neurosci. 2020 Jun;23(6):701-706. Epub 2020 Apr 27 PubMed.
- Chen WT, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, Qian X, Laláková J, Kühnemund M, Voytyuk I, Wolfs L, Mancuso R, Salta E, Balusu S, Snellinx A, Munck S, Jurek A, Fernandez Navarro J, Saido TC, Huitinga I, Lundeberg J, Fiers M, De Strooper B. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer's Disease. Cell. 2020 Aug 20;182(4):976-991.e19. Epub 2020 Jul 22 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Zeng H, Huang J, Zhou H, Meilandt WJ, Dejanovic B, Zhou Y, Bohlen CJ, Lee SH, Ren J, Liu A, Tang Z, Sheng H, Liu J, Sheng M, Wang X. Integrative in situ mapping of single-cell transcriptional states and tissue histopathology in a mouse model of Alzheimer's disease. Nat Neurosci. 2023 Mar;26(3):430-446. Epub 2023 Feb 2 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UK Dementia Research Institute@UCL and VIB@KuLeuven
This is a beautiful paper using spatial transcriptomics to depict the cellular phase of Alzheimer pathology (De Strooper and Karran, 2016). The technology brings us a huge step forward from our transcriptomic paper in 2020 (Chen et al., 2020). With the technology available to us at that time, we had a resolution of 100 µm allowing us to analyze small tissue domains. We could not perform protein staining on the same section and therefore used two adjacent slides to locate the amyloid plaques.
Zheng et al. provide real single-cell resolution combined with protein staining of amyloid plaques and tau pathology in situ. We feel very pleased to see that some of the major findings of our work hold upon this deeper scrutiny. The PIGs (plaque-induced genes) we described depict the microglia and astroglia responses around the plaques and their coordinated reaction to these pathologies, as seen here with disease-associated microglia and astrocytes. We also noticed in our paper the oligodendrocyte reaction contributing to the overall cell response, something that was a big surprise for us at that time because not many researchers were thinking about oligodendrocytes playing a role in the disease process.
It is clear that with this type of analysis, the field is transitioning into a new phase of understanding of the pathogenesis of Alzheimer’s disease. The emerging picture is that the amyloid deposits in the brains of mice and humans are not innocent. They induce a mainly microglia-driven multicellular response, most similar to a chronic inflammation. The fact that most of the risk genes for Alzheimer’s disease are expressed in microglia suggests that this response is central to the disease progression (Sierksma et al., 2020; Sierksma et al., 2020). It is not the amyloid plaque that causes the dementia, but the cellular response to these lesions that determines whether somebody becomes clinically sick or not.
References:
De Strooper B, Karran E. The Cellular Phase of Alzheimer's Disease. Cell. 2016 Feb 11;164(4):603-15. PubMed.
Chen WT, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, Qian X, Laláková J, Kühnemund M, Voytyuk I, Wolfs L, Mancuso R, Salta E, Balusu S, Snellinx A, Munck S, Jurek A, Fernandez Navarro J, Saido TC, Huitinga I, Lundeberg J, Fiers M, De Strooper B. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer's Disease. Cell. 2020 Aug 20;182(4):976-991.e19. Epub 2020 Jul 22 PubMed.
Sierksma A, Lu A, Mancuso R, Fattorelli N, Thrupp N, Salta E, Zoco J, Blum D, Buée L, De Strooper B, Fiers M. Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Mol Med. 2020 Mar 6;12(3):e10606. Epub 2020 Jan 17 PubMed.
Sierksma A, Escott-Price V, De Strooper B. Translating genetic risk of Alzheimer's disease into mechanistic insight and drug targets. Science. 2020 Oct 2;370(6512):61-66. PubMed.
Washington University School of Medicine
Washington University in St. Louis
Using in-house-developed STARmap PLUS for single-cell spatial transcriptomics, Zeng et al. established a cellular map that reflects disease pathology in the TauPS2APP transgenic model of Alzheimer’s disease. The beautiful data shown here nicely corroborated previous identifications of disease-associated microglia and astrocytes, and comprehensively provided a spatial relationship of these disease-associated populations with plaque and tau pathologies. The results showed that the STARmap PLUS technology can be widely used to study changes in gene signatures in 4D, combining space and time, through disease progression.
Compared to mouse models, spatial transcriptomic information from human patients is far sparser. STARmap PLUS will greatly aid in our understanding of disease progression when applied to postmortem human samples. For example, whether the core-shell structures are similarly presented in human tissues is unclear.
We and others have previously identified a reactive oligodendrocyte population in the 5XFAD mice and various CNS pathologies (Zhou et al., 2020; Shen et al., 2021; Kenigsbuch et al., 2022). This population was not highlighted in the current study, likely because one of the marker genes for this oligodendrocyte population, Serpina3n, was not included in the target gene list. Nonetheless, the Oligo2 cluster enriched in TauPS2APP might be the corresponding reactive oligodendrocytes. It is interesting to see that the Oligo2 cluster is enriched around p-tau but not as much around the amyloid plaques, suggesting differential effects of p-tau and amyloid on oligodendrocyte responses.
References:
Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR, Poliani PL, Cominelli M, Grover S, Gilfillan S, Cella M, Ulland TK, Zaitsev K, Miyashita A, Ikeuchi T, Sainouchi M, Kakita A, Bennett DA, Schneider JA, Nichols MR, Beausoleil SA, Ulrich JD, Holtzman DM, Artyomov MN, Colonna M. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease. Nat Med. 2020 Jan;26(1):131-142. Epub 2020 Jan 13 PubMed. Correction.
Shen K, Reichelt M, Kyauk RV, Ngu H, Shen YA, Foreman O, Modrusan Z, Friedman BA, Sheng M, Yuen TJ. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 2021 Mar 9;34(10):108835. PubMed.
Kenigsbuch M, Bost P, Halevi S, Chang Y, Chen S, Ma Q, Hajbi R, Schwikowski B, Bodenmiller B, Fu H, Schwartz M, Amit I. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat Neurosci. 2022 Jul;25(7):876-886. Epub 2022 Jun 27 PubMed.
Brigham & Women's Hospital
Brigham and Women's Hospital/Harvard Medical School
The authors present an improved spatial mapping method termed STARmap PLUS, which is able to capture both cell-specific transcriptional states and pathological hallmarks of Alzheimer’s disease (AD), such as Aβ plaques and neurofibrilarly tangles of tau. Using STARmap PLUS on the TauPS2APP mouse model (which exhibits both plaques and tangles), they highlight the enrichment of neurodegenerative microglia (MGnD or DAM) in immediate proximity of plaques, while disease-associated astrocytes (DAA) are frequent within the secondary perimeter. A disease-associated subtype of oligodendrocytes was found predominatly around tau-positive excitatory neurons in the CA1 region; however, this was predominantly at later stages (13 months compared to 8 months).
The study provides an important advance to our ability of mapping disease pathology with functional and identifiable cell states. STARmap PLUS outperforms current spatial expression methods, and reproduced key cell substates such as MGnD and DAA. The current limitation is the curated selection of 2,766 genes and the capture of about 70k cells. The technical constraint might explain why the recently identified interferon responsive microglia (IRM) subtype was not captured (Sala Frigerioet al., 2019; Dorman et al., 2022; Kaya et al., 2022).
Due to the two-dimesional restriction of the method, the spatial alterations to endothelial cells were also not captured. The importance of blood-brain barrier leakage, amyloid deposition in blood vessels, and cerebral amyloid angiopathy have been reported as major factors in AD (Kisler et al., 2017; Ellis et al., 1996; Johnson et al., 2007). Hence, associating particular changes in endothelial cells to local levels of amyloid deposition is of major interest to the the field. Furthermore, the technology holds the potential to highlight localized disease crosstalk, for example between MGnD and DAA around plaques.
Overall, STARmap PLUS shifts our understanding of individual cell substates themselves toward their simultanous pathological location, and future pertubation approaches will hopefully allow the identification of disease-promoting or -responsive mechanisms.
References:
Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen WT, Woodbury ME, Srivastava G, Möller T, Hudry E, Das S, Saido T, Karran E, Hyman B, Perry VH, Fiers M, De Strooper B. The Major Risk Factors for Alzheimer's Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep. 2019 Apr 23;27(4):1293-1306.e6. PubMed.
Dorman LC, Nguyen PT, Escoubas CC, Vainchtein ID, Xiao Y, Lidsky PV, Nakajo H, Silva NJ, Lagares-Linares C, Wang EY, Taloma SE, Cuevas B, Nakao-Inoue H, Rivera BM, Schwer B, Condello C, Andino R, Nowakowski TJ, Molofsky AV. A type I interferon response defines a conserved microglial state required for effective neuronal phagocytosis. bioRxiv. 2022 Feb 22; PubMed.
Kaya T, Mattugini N, Liu L, Ji H, Cantuti-Castelvetri L, Wu J, Schifferer M, Groh J, Martini R, Besson-Girard S, Kaji S, Liesz A, Gokce O, Simons M. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat Neurosci. 2022 Nov;25(11):1446-1457. Epub 2022 Oct 24 PubMed.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017 Jul;18(7):419-434. Epub 2017 May 18 PubMed.
Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology. 1996 Jun;46(6):1592-6. PubMed.
Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, Mathis CA, Price JC, Dekosky ST, Fischman AJ, Greenberg SM. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007 Sep;62(3):229-34. PubMed.
NYU Langone
Some technical thoughts: High-throughput in situ methods, such as STARmap and merscope, are going to continue to afford a greater degree of confidence in spatial transcriptomics methods moving forward. The ability to have individual cell resolution is a real bonus, and subcellular resolution is going to become the norm.
This is something that spotted oligo array systems, e.g. Visium, are unable to provide currently, though promised updates with VisiumHD may overcome some of these concerns with improved capture areas and greatly increased resolution. As a side note, however, if the fiducial area provides too many spots, we will be unable to afford the sequencing on these spots, as a predicted 1 million spots will be a huge cost barrier using current sequencing technology. Maybe Ultima or NOVAX from Illumina will fix this.
One remaining concern to keep in mind is that, while Visium provides lower fidelity, it does provide unbiased gene analysis without a requirement for a priori knowledge of “what to look for.” In situ based methods such as merscope and STARmap, while providing much higher resolution, require the selection of known marker genes to put into your panel of probes. This is less of a problem if people are completing paired sc/snRNAseq in experiments where they also take sections for spatial transcriptomics. I would also suggest doing this for Visium, so that you can make cell-type/sub-state specific modules of genes to probe back into the data.
Muna Therapeutics
We are happy to see that an amyloid-induced, multicellular, gene co-regulation network—i.e., 57 plaque-induced genes or PIGs that we identified in APPNL-G-F mice—has been cross-validated in multiple AD mouse models including 5XFAD, APPPS1, and now in TauPS2APP mice by Zeng and colleagues (Zhou et al., 2020; Kenigsbuch et al., 2022; Castranio et al., 2022). The results consistently show an activated glial niche surrounding amyloid plaques with APOE+/C1Q+ microglia as the core and GFAP+/C3+ astrocyte and SERPINA3+/C4B+/H2-D1+/B2m+ oligodendrocyte as the shell.
PIGs have been identified via different technologies and analyses. We did so via spatial transcriptomics validated by in situ sequencing (Chen et al., 2020). We performed spatial transcriptomics, i.e., 2D RNA-Seq array, to unbiasedly cluster genes with similar spatial expression pattern from a pool of more than 15,000 genes across more than 10,000 microenvironments at 100 micrometer resolution. Protein staining on tissue sections adjacent the spatial transcriptomics section indicated one network, i.e. PIGs, co-localized with amyloid plaque, and the strength of gene co-expression gradually increased with Aβ level. Therefore, these 57 PIGs are not only enriched around plaques, as identified in many papers, but also co-expressed across diverse microenvironments of brain regions, ages, and genotypes with varying degrees of amyloid stress. This indicates that PIGs function together as a multicellular mechanism against amyloid stress, relationships that could not be recognized using single-nuclei RNA-Seq or in situ technologies with low throughput and sensitivity.
We ran in situ sequencing to detect the 57 PIGs and many cell type markers, and to locate amyloid plaques via immunostaining on the same tissue section used for RNA-Seq, as did Zeng and colleagues. We thus validated enrichment of 57 PIGs around amyloid plaques at microscopic resolution in mouse brains and partially in human brains. However, due to the low sensitivity of in situ technology available at that time, we were unable to analyze gene expression at pseudo-cell resolution, or its distance to plaques. This paper nicely increases the throughput from 100 to 2,766 genes with good sensitivity, allowing pseudo-cell analysis and proximity to pathology. It’s a beautiful study to visualize the cellular response to amyloid plaques with single-cell and spatial resolution.
There is a trade-off between throughput, sensitivity, specificity, and resolution of spatial technologies. It’s exciting to see more and more technologies increasing throughput and sensitivity at microscope resolution to demultiplex RNA detection and become commercialized (He et al., 2021; Janesick et al., 2022; Chen et al., 2022).
Mouse models have contributed enormously to our understanding of AD; however, the utility of these mice as robust preclinical models has been challenged. Spatial omic technologies open the possibility to learn how cells function together against pathogenic hallmarks directly in human brains, and thus offer a fantastic platform to screen therapeutic targets of neurodegenerative diseases.
References:
Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR, Poliani PL, Cominelli M, Grover S, Gilfillan S, Cella M, Ulland TK, Zaitsev K, Miyashita A, Ikeuchi T, Sainouchi M, Kakita A, Bennett DA, Schneider JA, Nichols MR, Beausoleil SA, Ulrich JD, Holtzman DM, Artyomov MN, Colonna M. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease. Nat Med. 2020 Jan;26(1):131-142. Epub 2020 Jan 13 PubMed. Correction.
Kenigsbuch M, Bost P, Halevi S, Chang Y, Chen S, Ma Q, Hajbi R, Schwikowski B, Bodenmiller B, Fu H, Schwartz M, Amit I. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat Neurosci. 2022 Jul;25(7):876-886. Epub 2022 Jun 27 PubMed.
Castranio EL, Hasel P, Haure-Mirande JV, Ramirez Jimenez AV, Hamilton BW, Kim RD, Glabe CG, Wang M, Zhang B, Gandy S, Liddelow SA, Ehrlich ME. Microglial INPP5D limits plaque formation and glial reactivity in the PSAPP mouse model of Alzheimer's disease. Alzheimers Dement. 2022 Nov 30; PubMed.
Chen WT, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, Qian X, Laláková J, Kühnemund M, Voytyuk I, Wolfs L, Mancuso R, Salta E, Balusu S, Snellinx A, Munck S, Jurek A, Fernandez Navarro J, Saido TC, Huitinga I, Lundeberg J, Fiers M, De Strooper B. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer's Disease. Cell. 2020 Aug 20;182(4):976-991.e19. Epub 2020 Jul 22 PubMed.
He S, Bhatt R, Brown C, Brown EA, Buhr DL, Chantranuvatana K, Danaher P, Dunaway D, Garrison RG, Geiss G, Gregory MT, Hoang ML, Khafizov R, Killingbeck EE, Kim E, Kim TK, Kim Y, Klock A, Korukonda M, Kutchma A, Lewis ZR, Liang Y, Nelson JS, Ong GT, Perillo EP, Phan JC, Phan-Everson T, Piazza E, Rane T, Reitz Z, Rhodes M, Rosenbloom A, Ross D, Sato H, Wardhani AW, Williams-Wietzikoski CA, Wu L, Beechem JM. High-plex Multiomic Analysis in FFPE at Subcellular Level by Spatial Molecular Imaging. bioRxiv. July 19, 2022 bioRxiv
Janesick A, Shelansky R, Gottscho A, Wagner F, Rouault M, Beliakoff G, de Oliveira MF, Kohlway A, Abousoud J, Morrison C, Drennon TY, Mohabbat S, Williams S, 10x Development Teams, Taylor S. High resolution mapping of the breast cancer tumor microenvironment using integrated single cell, spatial and in situ analysis of FFPE tissue. bioRxiv. October 7, 2022 bioRxiv
Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, Qiu X, Yang J, Xu J, Hao S, Wang X, Lu H, Chen X, Liu X, Huang X, Li Z, Hong Y, Jiang Y, Peng J, Liu S, Shen M, Liu C, Li Q, Yuan Y, Wei X, Zheng H, Feng W, Wang Z, Liu Y, Wang Z, Yang Y, Xiang H, Han L, Qin B, Guo P, Lai G, Muñoz-Cánoves P, Maxwell PH, Thiery JP, Wu QF, Zhao F, Chen B, Li M, Dai X, Wang S, Kuang H, Hui J, Wang L, Fei JF, Wang O, Wei X, Lu H, Wang B, Liu S, Gu Y, Ni M, Zhang W, Mu F, Yin Y, Yang H, Lisby M, Cornall RJ, Mulder J, Uhlén M, Esteban MA, Li Y, Liu L, Xu X, Wang J. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell. 2022 May 12;185(10):1777-1792.e21. Epub 2022 May 4 PubMed.
Make a Comment
To make a comment you must login or register.