Inflammasomes Spread from Cell to Cell
Quick Links
Scientists may have discovered a new immune signaling mechanism. Back-to-back papers in the June 22 Nature Immunology report that inflammasomes, multiprotein complexes previously thought to be strictly intracellular, function outside the cell. Two independent groups of scientists, one led by Pablo Pelegrín, Clinical University Hospital Virgen de la Arrixaca, Murcia, Spain, the other led by Eicke Latz, University of Bonn, Germany, found that activating inflammasomes caused their release from macrophages, whereupon they continued to provoke inflammation in the extracellular milieu. Furthermore, when engulfed by neighboring macrophages, these particles triggered an inflammatory cascade in those cells.
“Now we can broaden the inflammasome repertoire to include acting as extracellular signals," Pelegrín told Alzforum. Scientists debate how important these new mechanisms are for health and disease. Given that Aβ has been shown to activate inflammasomes (see Dec 2012 news story), the findings might be relevant for Alzheimer's and potentially other neurodegenerative disorders.
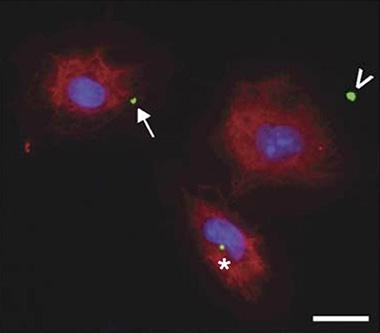
Go Forth and Multiply.
Inflammasome specks (green) move from inside (asterisk) the cell (red) near the nucleus (blue), to the edge (arrow), then to the outside (arrowhead), where they instigate more inflammation. Image courtesy of Baroja-Mazo et al. Nature Immunology.
Found in myeloid immune cells such as macrophages and brain glia, inflammasomes comprise complexes of proteins activated by a variety of extracellular signals to cause inflammation (for a review, see Schroder and Tschopp, 2010). The best-studied inflammasome is called nod-like receptor (NLR) family, pyrin domain-containing 3 type inflammasome, or NLRP3. When it senses a problem, such as reactive oxygen species or Aβ, NLRP3 oligomerizes and triggers assembly of a protein complex that goes by the long-winded name of apoptosis-associated speck-like protein containing a caspase-recruitment domain, or ASC. ASC bundles, called ASC specks, convert immature caspase-1 to its mature form, which in turn activates inflammatory interleukins IL-1β and IL-18. These then stream from the cell to attract neutrophils and monocytes. This process causes inflammatory-related cell death, called pyroptosis.
Both groups noticed inflammasome material in the extracellular space and wondered if it might play additional roles there.
Pelegrín, with co-first authors Alberto Baroja-Mazo and Fatima Martín-Sánchez, found that macrophages taken from mouse bone marrow spewed NLRP3 and ASC complexes into the extracellular space within 10 to 30 minutes of activation (see image above). Once outside, these particles processed and activated pro-caspase-1 and pro-IL-1β, which had also been released from the macrophage. These ASC specks were taken up by nearby macrophages, which then activated their own caspase-1, triggering release of more IL-1β. This whole process seemed to work in vivo, too. When injected into the peritoneum of wild-type mice, ASC specks raised the amount of extracellular IL-1β found there and recruited more white blood cells to the injection site.
Pelegrín’s group examined this process with various mutant forms of NLRP3. Some of these mutations have been linked to cryopyrin-associated periodic syndromes (CAPS), a type of autoinflammatory disorder. In cells with the D303N substitution, NLRP3 spontaneously aggregated. Outside the cells, this mutation caused ASCs to oligomerize into specks, which macrophages then engulfed, perpetuating an inflammatory cascade.
Latz and colleagues found something very similar. Using fluorescent antibody staining, first author Bernardo Franklin saw that after pyroptosis, ASC specks built up outside human monocytes. The ASC specks recruited and activated both caspase-1 and IL-1β in the extracellular space. Macrophages then ingested these specks, causing their lysosomes to swell and leak, activating their own inflammasomes and causing endogenous ASC to aggregate, forming more specks. The researchers likened this cell-to-cell spread of ASC specks to the “seeding” activity of prion-like proteins.
To check for a similar process in vivo, Latz and his group injected fluorescently labeled ASC specks into the skin of mice and watched for the recruitment of neutrophils. Compared to plain fluorescent beads, the ASC specks attracted more of the immune cells, suggesting they caused the release of more IL-1β, which attracts neutrophils. Injecting Pseudomonas bacteria into the mice’s footpads also promoted the appearance of ASC specks outside cells.
Could these findings be relevant to human disease? Pelegrín found that ASC specks were more abundant in the serum of people diagnosed with CAPS than in serum from healthy controls. Similarly, Latz’s group found extracellular ASC specks accumulating in the lungs of patients with chronic obstructive pulmonary disease, an inflammatory lung disorder that might be related to inflammasomes.
It is unclear what these findings mean for neurodegenerative diseases, said Pelegrín. He is studying how this process could relate to AD and related disorders by using animal models. Latz plans to do the same. Charles Dinarello, University of Colorado School of Medicine, Aurora, thinks this process could occur in the brain where it may contribute to Alzheimer’s and other neurodegenerative disorders. Seth Masters, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia, believes this process could provide a drug target. “Therapies could be designed to shut down this amplification of inflammation,” he wrote in an email.
Dave Morgan, University of South Florida, Tampa, was less convinced that extracellular specks contribute to neurodegenerative diseases. While there is some evidence that Aβ activates inflammasomes, Morgan doubts it is enough to generate ASC specks. “I suspect that activation of ASC specks is too severe to be a major participant in slow, progressive neurodegenerative diseases,” he said. On the other hand, it could be part of the secondary damage that follows stroke or severe brain trauma. In general, this is likely another way that the immune system cranks up its positive feedback system, and a possible target for therapeutic intervention for some forms of inflammation, Morgan said.
How is this process quenched after immune cells have successfully neutralized a threat? Pelegrín is studying that now.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010 Mar 19;140(6):821-32. PubMed.
Further Reading
Papers
- Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014 Jul;14(7):463-77. PubMed.
- Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, Broadhurst DI, Power C. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. 2014 May 13;11:35. PubMed.
- Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014 May 22; PubMed.
Primary Papers
- Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, Buján S, Couillin I, Brough D, Arostegui JI, Pelegrín P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014 Jun 22; PubMed.
- Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmüller W, Latz E. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat Immunol. 2014 Aug;15(8):727-37. Epub 2014 Jun 22 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The Walter and Eliza Hall Institute
These two recent papers in Nature Immunology provide clear evidence that an intracellular protein complex known as the inflammasome can actually be released from the cell after activation. This is potentially relevant in the context of neurodegenerative disease, as one particular inflammasome complex, regulated by the protein NLRP3, is thought to contribute to the progression of Alzheimer's, amyotrophic lateral sclerosis, and other neuroinflammatory conditions.
In the context of infection, this protein complex is very important for inflammation that can defeat pathogens, however, it is somehow being activated inappropriately in the context of neurodegeneration. These new findings show that not only does the initial cell that gets activated produce inflammatory cytokines, but that the actual protein complex itself is released, propagating inflammation in nearby cells, and also systemically.
This discovery now provides a rationale for therapies that could shut down this amplification of inflammation, because previously it was not possible to target the intracellular protein complex with neutralising antibodies. However, preliminary studies in this paper show that using the wrong type of antibodies to target this extracellular protein complex does not actually neutralise it, and instead it can promote uptake and actually increase inflammation. Fortunately, the technology exists to develop antibodies that will not be taken up in this manner, and it should be possible to neutralize this pathway in the near future.
Make a Comment
To make a comment you must login or register.