Introducing LATE—A Common TDP-43 Proteinopathy that Strikes After 80
Quick Links
An 86-year-old woman develops classic amnestic symptoms of Alzheimer’s disease in her last years of life, but upon autopsy, her brain bears but a modest burden of Aβ plaques and tau tangles. Instead, TDP-43 inclusions crowd her limbic regions. This would be a typical case of limbic-predominant age-related TDP-43 encephalopathy (LATE), a common neurodegenerative disease christened by an international cadre of pathologists, clinicians, and epidemiologists in a consensus report published April 30 in Brain. They predict the disease will account for one in five clinical AD diagnoses. Its pattern of TDP-43 pathology is at least somewhat distinct from that observed in frontotemporal dementia (FTD). In their paper, drafted following a workshop in Atlanta last fall, researchers led by Peter Nelson of the University of Kentucky in Lexington summed up decades of research leading up to the coining of the term. They describe LATE’s features, propose ways to classify and diagnose it, and call for the development of specific biomarkers and therapeutics.
- LATE: Limbic-predominant age-related TDP-43 encephalopathy.
- Distribution of TDP-43 pathology is distinct from FTD.
- Clinical features are similar to those of Alzheimer’s.
Carol Brayne of the University of Cambridge, U.K., is one of the paper’s 35 co-authors. She said the report may help shift the dementia field away from its focus on AD neuropathology, a move she considers crucial in light of the mixed pathological jumble often present in the oldest brains upon autopsy. “For too long, millions of dollars have been chasing an idea of mechanisms with elegant research that is aimed at an assumed pathology, instead of looking at what is really there in the population. This paper helps to shift the narrative,” Brayne wrote to Alzforum.
Other researchers in the field expressed reservations about designating LATE—primarily a neuropathological entity—as its own disease. “The construct of LATE will give TDP-43 the recognition that it deserves and hopefully bring TDP-43 to the forefront of Alzheimer’s disease research,” commented Keith Josephs of the Mayo Clinic in Rochester, Minnesota. “That said, LATE is not a new disease. It may not even be a disease, given that 20 percent of individuals who die with normal cognition will have LATE. Instead, LATE is a rebranding of science, a catchy acronym, but if you can get by all the hype it is really just TDP-43 in the brains of old people, including those with plaques and tangles.”
Relatively few people make it into their 80s without some form of neuropathology arising in their brain, and mixed pathology is the norm. Neuropathologists commonly observe cytoplasmic inclusions of phosphorylated TDP-43 in postmortem brain samples with or without the Aβ plaques and neurofibrillary tangles that define AD.
Inclusions containing this RNA-binding protein were first implicated in ALS and FTD more than a decade ago (Neumann et al., 2006; Cairns et al., 2007). Soon after, neuropathologists led by Dennis Dickson at the Mayo Clinic in Jacksonville, Florida, spotted TDP-43 pathology in people who had had symptoms of AD, not FTD (Amador-Ortiz et al., 2007). It often accompanied hippocampal sclerosis—a shrunken hippocampus ravaged by gliosis. More than a decade prior, Dickson had described curious cases of hippocampal sclerosis in people with dementia without much AD pathology (Dickson et al., 1994).
Researchers now suspect that a large proportion of those cases, as well as cases more recently classified as suspected non-Alzheimer’s disease pathophysiology (SNAP), are caused by TDP-43 pathology (Sep 2015 conference news; Cykowski et al, 2017).
In the past decade, several studies have described the clinical and pathological course of this TDP-43 proteinopathy, newly dubbed LATE. One measured TDP-43 pathology in postmortem brain samples from nearly 1,000 deceased participants in the Rush Memory and Aging Project and Religious Orders Study (ROS-MAP) (James et al., 2016). Half of the participants had TDP-43 pathology, and 37 percent had both TDP-43 and AD pathology. People with both were more likely to have had clinical dementia than those who had either. This study proposed a pathological staging scheme, in which TDP-43 pathology hits the amygdala in stage 1, then the hippocampus and entorhinal cortex in stage 2, and finally the neocortex in stage 3. Once the pathology extends beyond the amygdala, it correlates with cognitive impairment.
The consensus report suggests this scheme for neuropathological staging of LATE, citing MRI support for the idea that the amygdala and hippocampal regions are affected in people who were later confirmed to have LATE.
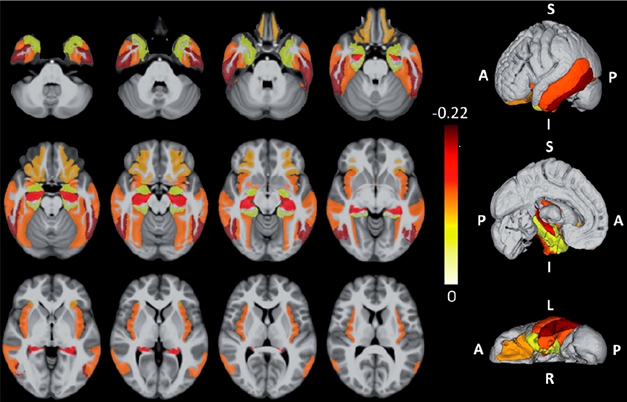
LATE’s Mark on the Brain. LATE-specific atrophy patterns averaged from ROSMAP brain samples that were later confirmed to have LATE. Darker colors represent more atrophy. Rows are different brain views; each displayed with four different cut points. [Courtesy of Nelson et al., Brain 2019.]
LATE-associated TDP-43 pathology often co-occurs with hippocampal sclerosis, but the latter is not a requirement for a neuropathological diagnosis of LATE. Compared with the pattern of TDP-43 pathology seen in FTD—which also affects the hippocampus and neocortex—that observed in LATE leans more limbic, with less cortical atrophy, and crops up in older adults (Nelson et al., 2011).
What are clinical manifestations of this proposed new disease? The authors say LATE has a lot in common with AD. Episodic memory wanes early, other cognitive domains later. When this happens without substantial AD pathology, the memory loss is more gradual and less severe than in AD (Boyle et al., 2017; Nag et al., 2017). However, in people who also have abundant Aβ plaques and tau tangles, LATE neuropathology exacerbates cognitive symptoms beyond what would be expected for AD alone (Josephs et al., 2015).
Given the clinical ambiguity of LATE, Ian Mackenzie of the University of British Columbia in Vancouver, Canada, who was not involved in drafting the report, thinks it should have been named based on its pathology instead. “The authors propose the term LATE for a disease/condition that has no criteria other than the associated pathology,” Mackenzie noted. “It would be more logical to come up with a new name for the pathology, for example Limbic Predominant Age-related TDP-43 Proteinopathy, and then propose the existence of an LPATP-associated clinical syndrome which still needs to be defined,” he wrote to Alzforum. Mackenzie also noted that pathological differences between LATE and FTLD-TDP still need to be addressed.
Manuela Neumann of the German Center for Neurodegenerative Diseases in Tübingen raised similar issues. “How useful is the introduction of a new disease that is defined and can be diagnosed so far only by neuropathological findings obtained postmortem?” she wrote.
How common is LATE? ROSMAP researchers, led by Julie Schneider at Rush, reanalyzed data from their cohort to account for its proposed neuropathological staging. The consensus paper notes that this pathology was detected in one in five brains from people older than 80, extrapolating that 15 percent to 20 percent of clinically diagnosed AD cases at that age are attributable to LATE. They estimate the impact of LATE at about half that of AD in old people, and equal to the combined impact of all vascular neuropathologies. This would make LATE about 100 times more prevalent than FTD.
The report summarized data implicating genes in LATE neuropathology or hippocampal sclerosis, naming risk alleles in GRN, TMEM106b, ABCC9, KCNMB2, and APOE.

LATE by Elimination? Two cases of dementia without one or both of the hallmark AD pathologies (A=amyloid, T=tau), but with marked hippocampal neurodegeneration (N). These could be cases of LATE. [Courtesy of Nelson et al., Brain, 2019.]
With the proposed new disease’s clinical resemblance to AD, how will researchers tell the two apart during life? No fluid biomarkers or PET tracers exist to detect TDP-43 pathology, though researchers are working on the problem, Nelson said. TDP-43’s intracellular location and small pathological burden make biomarker development challenging. For now, the best a diagnostician can do is follow a process of elimination. For example, using the AT(N) system for classifying AD where amyloid=A, tau=T, and neurodegeneration=(N), they might suspect LATE neuropathology in people who are A-T-N+, or A+T-N+ (Aug 2016 news). FDG-PET scans detect flagging metabolism in the medial temporal lobe in people with negative tau PET scans who later were found to have LATE neuropathology (Botha et al., 2018).
Even when AD pathology co-exists, specific atrophy patterns might be able to pick up LATE neuropathology, co-author John Trojanowski of the University of Pennsylvania in Philadelphia pointed out. He noted that UPenn researchers led by Christos Davatzikos have developed algorithms to detect AD and PD-specific atrophy patterns in MRI data, and suggested similar algorithms could be developed for LATE (Davatzikos et al., 2009).
“The full range of TDP-43 pathology across the whole brain in relation to dementia in the population has not yet been fully described,” noted consensus co-author Sally Hunter of the University of Cambridge, U.K. “This lack of clarity coupled with a lack of biomarkers specific for TDP-43 leads to difficulties in selecting well-defined and meaningful cases and controls both for research and for randomized controlled trials,” she wrote.
The co-occurrence of AD and LATE neuropathology in old age complicates interpretation of AD-specific treatment trials, and may even obscure positive results, Nelson noted. Until specific biomarkers for LATE neuropathology exist, clinical trials will need to be powered to account for TDP-43 proteinopathy, the authors wrote.
Once biomarkers exist, they will facilitate drug-discovery efforts targeted at LATE, for example at its TDP-43 aggregates. Given that comorbid neuropathologies in people at risk for LATE could potentially interact (Robinson et al., 2018), Trojanowski proposed an immunotherapeutic cocktail of antibodies aimed at Aβ, tau, TDP-43, and α-synuclein.—Jessica Shugart
References
News Citations
- Suspected Non-Alzheimer Pathophysiology: It’s Not Exactly a Snap
- Staging of Alzheimer’s, the Second: Neurodegeneration Does Not Equal Tauopathy
Paper Citations
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006 Oct 6;314(5796):130-3. PubMed.
- Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007 Jul;171(1):227-40. PubMed.
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007 May;61(5):435-45. PubMed.
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88(3):212-21. PubMed.
- Cykowski MD, Powell SZ, Schulz PE, Takei H, Rivera AL, Jackson RE, Roman G, Jicha GA, Nelson PT. Hippocampal Sclerosis in Older Patients: Practical Examples and Guidance With a Focus on Cerebral Age-Related TDP-43 With Sclerosis. Arch Pathol Lab Med. 2017 Aug;141(8):1113-1126. Epub 2017 May 3 PubMed.
- James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016 Sep 30; PubMed.
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011 May;134(Pt 5):1506-18. PubMed.
- Boyle PA, Yang J, Yu L, Leurgans SE, Capuano AW, Schneider JA, Wilson RS, Bennett DA. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017 Mar 1;140(3):804-812. PubMed.
- Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology. 2017 Feb 14;88(7):653-660. Epub 2017 Jan 13 PubMed.
- Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, Petrucelli L, Senjem ML, Ivnik RJ, Parisi JE, Petersen RC, Dickson DW. TAR DNA-binding protein 43 and pathological subtype of Alzheimer's disease impact clinical features. Ann Neurol. 2015 Nov;78(5):697-709. Epub 2015 Sep 16 PubMed.
- Botha H, Mantyh WG, Murray ME, Knopman DS, Przybelski SA, Wiste HJ, Graff-Radford J, Josephs KA, Schwarz CG, Kremers WK, Boeve BF, Petersen RC, Machulda MM, Parisi JE, Dickson DW, Lowe V, Jack CR Jr, Jones DT. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain. 2018 Apr 1;141(4):1201-1217. PubMed.
- Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer's-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain. 2009 Aug;132(Pt 8):2026-35. PubMed.
- Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM, Trojanowski JQ. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018 Jul 1;141(7):2181-2193. PubMed.
Further Reading
Papers
- Katsumata Y, Fardo DW, Kukull WA, Nelson PT. Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer's disease and cerebrovascular disease pathologies. Acta Neuropathol Commun. 2018 Dec 19;6(1):142. PubMed.
- Jack CR Jr, Knopman DS, Chételat G, Dickson D, Fagan AM, Frisoni GB, Jagust W, Mormino EC, Petersen RC, Sperling RA, van der Flier WM, Villemagne VL, Visser PJ, Vos SJ. Suspected non-Alzheimer disease pathophysiology--concept and controversy. Nat Rev Neurol. 2016 Feb;12(2):117-24. Epub 2016 Jan 18 PubMed.
Primary Papers
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist IT, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SR, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, White Iii CL, Yu L, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019 Jun 1;142(6):1503-1527. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Tübingen and DZNE AG Neumann
This is a thoughtful summary of the numerous reports and findings over the last decade on TDP-43 pathology restricted to the limbic system common in elderly patients and often associated with hippocampal sclerosis and/or Alzheimer’s disease. While this paper is thought-provoking and important to promote research and awareness of this type of pathology and comorbidities, I also have the following concerns.
Mayo Clinic
TDP-43 was first discovered in 2006 as a ubiquitinated protein associated with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Since then it has been found to be associated with many other diseases, including Alzheimer’s.
I have been involved with TDP-43 research for over a decade, having been the one who first demonstrated that TDP-43 is indeed associated with memory loss in Alzheimer’s disease, way back in 2008 (Josephs et al., 2008). I do believe that TDP-43 is a very important protein, equal to, or greater than, amyloid and tau in importance. TDP-43 has been under-recognized by an Alzheimer’s establishment that can’t seem to get beyond amyloid and tau. The construct of LATE will give TDP-43 the recognition that it deserves and hopefully bring TDP-43 to the forefront of Alzheimer’ disease research.
That said, LATE is not a new disease. It may not even be a disease, given that 20 percent of individuals who die with normal cognition will have LATE. Instead, LATE is a rebranding of science, a catchy acronym, but if you can get by all the hype, it is really just TDP-43 in the brains of old people, including those with plaques and tangles.
References:
Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CR, Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008 May 6;70(19 Pt 2):1850-7. PubMed.
Cambridge University
From my perspective as an epidemiologist who has sought to lead studies that provide a platform and insight into what dementia really is in the true population, this work has been incredibly important, and at last begins to move us on from the narrow focus of traditional Alzheimer’s disease neuropathology and their mechanisms.
It’s clear there is so much more, particularly for the older old populations. For too long, millions of dollars have been chasing an idea of mechanisms with elegant research that is aimed at an assumed pathology instead of looking at what is really there in the population. This paper helps to shift the narrative.
In the older population amongst whom most dementia occurs, the relationships between clinically defined dementia and the variety of neuropathologies seen in the brain have long been acknowledged to be complex. There are those with very little pathology and dementia and those with severe pathology and no dementia. Additionally, the majority of those with dementia in the older population have mixed neurodegenerative and vascular pathologies, making the diagnosis of specific dementia-causing processes difficult.
Beyond the traditional focus on tau and Aβ, TDP-43 is increasingly being seen as an important contributor to the risk of developing dementia. As with the Alzheimer's disease-associated tau and Aβ pathologies, TDP-43 is seen in those with and without dementia. The full range of TDP-43 pathology across the whole brain in relation to dementia in the population has not yet been fully described.
This lack of clarity, coupled with a lack of biomarkers specific for TDP-43, leads to difficulties in selecting well-defined and meaningful cases and controls both for research and for randomized controlled trials.
The LATE consensus working report highlights again the complex presentation of dementia in the human population and focuses attention on the need for better research in humans to characterize the wide range of neuropathologies and their contributions to dementia.
University of Kentucky
Hi, a few quick comments ...
1. The consensus working group report on LATE was that—a distillation of what an international, multidisciplinary group could agree on.
2. The goal was not to produce a trendy new term. For me personally, a key goal was to have a diagnosis that could be made in a meaningful way. For example, at our center, we just signed out nine cases and four demonstrated what I can now call LATE-NC. It is not meaningful (or rather, it is needlessly puzzling) to patients, clinicians, or anybody else when different diagnosticians apply completely different terms and criteria for a common phenomenon. The best e-mail I got in the past few days was from a respected colleague who wrote, "I already used LATE as a diagnosis today!"
3. This was not a paper about FTD/FTLD. The commonalities and differences related to LATE-NC and FTLD-TDP were, naturally, a point of discussion among the group. Without getting down in the weeds, a consensus was not reached on that. Here are some differences that were agreed on and are highlighted in the paper: LATE-NC is ~100-fold more prevalent than FTLD-TDP (lifetime risk is 1:4 versus ~1:1000); LATE-NC tends to affect people in a quantumly older age group; persons with FTLD-TDP tend to have language and/or behavioral differences that have not been described in LATE; rather, persons with LATE-NC at autopsy tended to have deficits in episodic memory. I, personally, would guess that those differences will be shown to be correlated with differences in the neuropathology, and other parameters, but time will tell. MAPT haplotypes make all sorts of tauopathies worse; doesn't make them all FTLD-Tau. Just as not all tauopathies are AD, not all TDP-43 proteinopathies are FLTD/ALS.
4. This is a massively underappreciated and understudied disease(s)—and yes, TDP-43 proteinopathy (with or without comorbid AD pathology) is strongly associated with cognitive impairment, although, for a gradually progressive disease that affects >85-year-olds preferentially, a lot of persons die in a presumed preclinical state.
It is of course true that this is not the first word on age-related TDP-43 proteinopathy, nor, of course, the last. However, this is the first consensus group effort to address this topic, and it is hoped that this paper will help to move the field forward.
IRCCS FBF
It should be considered that the TDP-43 discovered pathological entity could be the neuropathological substrate of accelerated-forgetting syndromes of newly learned information, in particular in transient epileptic amnesia (TEA) and SNAP.
Make a Comment
To make a comment you must login or register.