Longevity Through Gut Bacteria? It Works in Worms
Quick Links
Through the ages, the quest for longevity has taken alchemists and scientists to unusual places. A present-day foray led Meng Wang at Baylor College in Houston to explore the jungle of genes inside a bacterium that lives in the gut of a tiny worm. In the June 15 issue of Cell, Wang and colleagues report finding 29 Escherichia coli genes that, when deleted, lengthen the lives of their Caenorhabditis elegans hosts. The study also shows that 14 of these mutations help keep Aβ-expressing worms moving and living longer. Unraveling the mechanism underlying five of the mutations, the scientists found that the bacterial polysaccharide colanic acid lengthened the worm’s life by modulating mitochondrial signaling in their cells.
“It’s a brilliant paper, a milestone in the field. Combining the genetic versatility of the worm with the accessibility of E. coli genetics is very powerful,” said Dario Valenzano at the Max Planck Institute in Cologne, Germany, who recently extended the lifespan of middle-aged fish by re-colonizing their guts with bacteria from young fish (Smith et al., 2017).
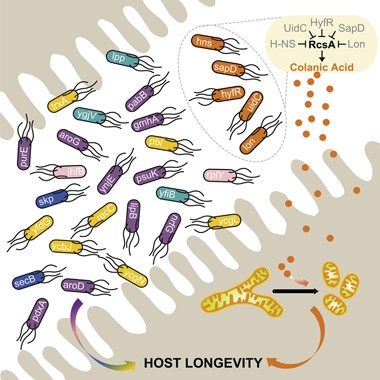
Gotta love your gut bugs.
Twenty-nine E. coli mutants increase longevity while teeming in the C. elegans intestine. Five (orange) boost colanic acid, a polysaccharide that triggers mitochondrial fragmentation. [Cell, Han et al., 2017.]
Microbial gut-dwellers influence their hosts in many ways. They churn out essential metabolites, process food, and biochemically modify host metabolites. And a growing number of conditions, including aging and neurodegenerative disease, seem to be influenced by these bacterial communities. Experiments in C. elegans, a workhorse in the field of aging research, have pointed to bacterial variants and small molecules they secrete, such as non-coding RNAs and nitric oxide, as modulators of the worm’s lifespan (Heintz and Mair, 2014).
So far, no one had conducted a systematic, genome-wide search for bacterial genes that influenced C. elegans longevity. To do so, first author Bing Han screened a library of 3,983 E. coli mutants, each lacking a single, non-essential gene, for their effects on C. elegans. Twenty-nine mutants prolonged the worms’ lifespan by more than 10 percent. Top performer hns, which encodes a transcriptional regulator, made the worms live 40 percent longer. Many of the mutants were unable to prolong the lives of worms that already carried mutations in genes previously known to regulate lifespan, such as daf-16, rsks-1, raga-1, rict-1, or a mutation that mimics caloric restriction, suggesting they act through these pathways.
To see if these life-extending bacterial mutations affect age-related pathologies such as cancer and neurodegeneration, the researchers tested them in tumor-prone C. elegans mutants and in Aβ-expressing transgenic worms. The latter were developed by Chris Link at the University of Colorado in Boulder; they express Aβ42 under the control of the muscle-specific unc-54 promoter/enhancer (Link, 1995). Fourteen of the bacterial mutants prolonged the lives of these transgenic worms by 10 to 40 percent; 12 delayed their age-associated paralysis (see image). Interestingly, 13 of the 14 life-extending mutants also prolonged the lives of tumor-ridden worms.
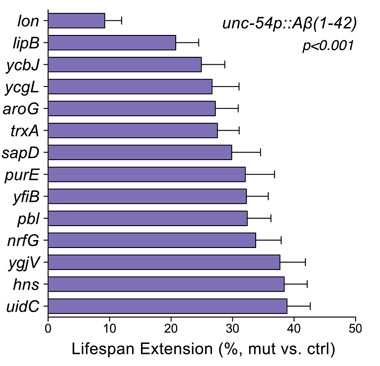
Lease on life.
Fourteen bacterial genes, when deleted, can extend the lifespan of C. elegans hosts expressing human Aβ in muscle cells. [Cell, Han et al., 2017.]
Link praised the findings but cautioned that the paralysis in his worms confounds survival measurements. For example, some females die because their paralyzed vulval muscles render them unable to lay their eggs. He also noted that the transgenic protein, expressed only in muscle cells, forms mostly intracellular inclusions, rather than typical extracellular plaques.
Wang and colleagues then focused on two bacterial mutants, Δlon and Δhns, that extended lifespan in normal and Aβ-expressing worms, but did so independently of the known longevity pathways. They noticed that both lon and hns encode proteins that reduce levels of colanic acid (CA). Secreted by many gut bacteria, this polysaccharide contains a repeating unit of glucose, galactose, fucose, and glucuronic acid, decorated by pyruvate and acetate. Wang found that three other bacterial mutants also overproduce CA; they not only extend lifespan but dampen the mitochondrial fragmentation normally seen in aging body-wall muscles.
To test whether CA was indeed an anti-aging elixir, Wang’s team added purified CA to wild-type C. elegans. Though less potent than the mutations, a little dietary CA extended the lives of worms grown on a variety of bacteria. It made old worms move faster, and once again prolonged the lives of tumor-prone and Aβ-expressing worms. Even a distantly related species, Drosophila melanogaster, benefited from eating CA. The flies lived longer and were more active later in life than controls.
Probing how CA accomplishes this, the authors searched for pathways that, when mutated in C. elegans, would cancel out CA’s benefit. Indeed, two mutations that disrupt the mitochondrial electron transport chain negated CA’s effects. Wang and her colleagues also observed that mitochondria in the intestinal cells of worms eating CA were more fragmented than those of non-treated controls. This CA-induced splintering of mitochondria also happened in a mammalian fibroblast cell line.
Even though mitochondrial fragmentation in aging muscles of the worm’s body wall is considered a sign of age-associated atrophy, the researchers reasoned that the CA-induced fragmentation in gut cells and fibroblasts could reflect healthy cells ramping up a quality-control mechanism that rids cells of damaged or dysfunctional mitochondria. Drp-1 is a gene required for mitochondrial fission and linked with increased longevity. To test this, the authors added CA to drp-1 mutants, and as expected, CA was no longer able to promote longevity. Drilling deeper, the researchers found that in mutants with compromised mitochondrial protein folding, CA triggered a strong mitochondrial stress response, activating the unfolded protein response (UPRmt) via the ATFS-1 transcription factor.
“They dissected down to the mechanism in both the bug and the host,” said Valenzano. “They can recapitulate the effect with the metabolite and show that it’s robust in other species. It’s a super-strong story.”
Does CA help cells beyond the gut? “We think the major active site is the gut,” said Wang. The authors found that CA requires host endocytic genes rab-5 and rab-7 to lengthen lifespan, suggesting the intestinal cells need to endocytose CA for it to work. “The gut then generates second messengers that could reach distant tissues, like muscle.” Other studies show that inducing the UPRmt in one tissue can induce a mitochondrial stress response in a distant tissue (Durieux et al., 2011). “Mitochondria are known to be important mediators of long-distance signals,” said Wang.
What’s next for CA? Many unknowns remain. Culture bacteria produce CA in response to stress and biofilm formation, but how and when it is made in vivo is unknown, said Wang. The length of the polymers, and how much of it bacteria make and consume, are nagging questions, she added, because the bacterial mutants that overproduce CA are twice as effective at lengthening lifespan as the purified polymer. “We are fractionating CA based on molecular weight,” said Wang. This will help pinpoint the active ingredient(s) and enable dosing studies.
The scientists want to test CA in mammals. “We need to purify a lot to test in mice,” said Wang. “We are also working on attaching a particle to track it in vivo.” Asked if she has tried CA herself, Wang said, “We joke in the lab: Are you secretly taking it?” Turning serious, Wang said CA could become an appealing supplement because, given its source, she suspects it likely will be safe.
Does this work have implications for neurodegenerative disease? Taoufiq Harach of Reminisciences, a biotechnology company in Paris focusing on microbe-based treatment and diagnosis for Alzheimer’s disease, said he would have liked to see the effect of the life-extending mutants and CA on amyloid. He also noted the importance of examining other AD models that more closely mirror the human disease.
Link noted that the mitochondrial-CA connection fits with his unpublished observation that shifting the balance of mitochondrial fission versus fusion toward fission made Aβ-expressing worms more mobile. Knocking down drp-1 reduced mitochondrial fission, as expected, but also increased paralysis, whereas knocking down EAT-3, a gene required for mitochondrial fusion, got the worms moving. Link added that many mitochondria in the muscle cells are misshapen and depolarized, and he suspects amyloid may be poking holes in them. If so, increasing the organelle’s turnover may be beneficial. It is unclear how this scenario would unfold in the brain.
Researchers have drawn connections between gut bacteria and neuropathology. Microbiome changes have been reported early in neurodegeneration (e.g., Apr 2017 news; Dec 2016 news), and several studies have connected the microbiome to inflammation and neurodegeneration. One recent study linked brain amyloidosis to bacteria with known pro-inflammatory effects, while bacteria with known anti-inflammatory effects were reduced in elderly people with cognitive impairment (Cattaneo et al., 2017). There are also reports of bacterial short-chain fatty acids modulating inflammation.
Looking ahead at future clinical applications, some researchers are betting on probiotics while others are hunting down specific metabolites. Harach favors the former. He thinks isolating specific molecules and finding the right dosing will take more time and money than tweaking a person’s microbiome. He claimed to have identified bacterial strains that protect mice against amyloid; his company is looking for funding to begin a clinical trial. In a recent study, researchers laced the drinking water of 3xTg-AD mice with a cocktail of lactic acid bacteria and bifidobacteria. The treated mice had less amyloid aggregates and better performance on behavior assays than those drinking plain water. The authors suggest the effect may be mediated by partial restoration of proteasome and autophagy function (Bonfili et al., 2017).
On the other hand, Ullrich Wüllner at the University of Bonn in Germany is seeking specific bacterial molecules to help patients with Parkinson’s disease. He is interested in short-chain fatty acids and is conducting metabolomic analysis to find other therapeutic candidates, including anti-inflammatory compounds.
For her part, Wang’s team is trying to generate probiotic strains of the other longevity-promoting mutants for testing in mammals. She sees advantages in both approaches. Working with specific compound allows for targeted, mechanistically based interventions, but probiotics may have broad beneficial effects arising from the joint activities of multiple compounds that could be difficult to tease apart.—Marina Chicurel
References
News Citations
- Rumblings of Parkinson’s: Gut Microbiome Shifts in Early Stage of Disease
- Do Microbes in the Gut Trigger Parkinson’s Disease?
Research Models Citations
Paper Citations
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of Life Span by the Gut Microbiota in The Short-Lived African Turquoise Killifish. bioRχiv. April 6, 2017
- Heintz C, Mair W. You are what you host: microbiome modulation of the aging process. Cell. 2014 Jan 30;156(3):408-11. PubMed.
- Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9368-72. PubMed.
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011 Jan 7;144(1):79-91. PubMed.
- Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB, INDIA-FBP Group. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017 Jan;49:60-68. Epub 2016 Aug 31 PubMed.
- Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, Fiorini D, Boarelli MC, Rossi G, Eleuteri AM. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017 May 25;7(1):2426. PubMed.
Further Reading
Papers
- Main BS, Minter MR. Microbial Immuno-Communication in Neurodegenerative Diseases. Front Neurosci. 2017;11:151. Epub 2017 Mar 23 PubMed.
Primary Papers
- Han B, Sivaramakrishnan P, Lin CJ, Neve IA, He J, Tay LW, Sowa JN, Sizovs A, Du G, Wang J, Herman C, Wang MC. Microbial Genetic Composition Tunes Host Longevity. Cell. 2017 Jun 15;169(7):1249-1262.e13. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Massachusetts General Hospital & Harvard Medical School
These findings add to rapidly mounting data that point to a role for micro-organisms in Alzheimer's disease (AD). Recent findings from both mouse models and human nutrition studies suggest the gut microbiome modulates brain β-amyloidosis (Minter et al., 2016). Here, Han et al. suggest the microbial polysaccharide colanic acid may mediate the beneficial host outcomes that appear associated with specific gut microbes, including ameliorating the harmful effects of Aβ fibrillization.
These findings are consistent with the emerging antimicrobial protection hypothesis for AD that we (Kumar et al., 2016), and others (Bourgade et al., 2016), recently proposed. In this model, Aβ is an antimicrobial peptide (AMP) of innate immunity that protects against infecting pathogens and, pertinent to the Han et al. study, microbial products. Fibrillization mediates Aβ’s antimicrobial activities and leads to protective sequestration of pathogens or microbial endotoxins in β-amyloid deposits (Kumar et al., 2016). Aβ contains an AMP heparin-binding motif (XBBXBX) that mediates microbial targeting and binding (Kumar et al., 2016). Soluble host sugars inhibit microbial binding of Aβ and other AMPs, regulating their activities. However, bacteria also secret exopolysaccharides that target AMPs and inhibit their activities, including polysaccharides that specifically inhibit microbial agglutination.
Aβ microbial agglutination pathways lead to generation of β-amyloid. Colanic acid and other exopolysaccharides, as well as a plethora of additional secretory products, are generated by the gut microbiome and enter the CNS, either from the periphery or via the vagus nerve. A key role for AMPs in the immunoprivileged brain is the agglutination and neutralization of potentially harmful microbial products. We suspect this may also emerge as a key innate immune role for Aβ in brain. Thus, the antimicrobial protection model provides a plausible pathway in which microbial molecules bind to Aβ, directly modulating β-amyloid deposition. Importantly, the microbial molecules need not be generated in the brain, but can be translocated from the gut microbiome or sites of infection.
The protective effect of colanic acid observed by Han et al in Aβ-expressing transgenic CL2006 nematodes is consistent with this model. It is worth noting that the transgenic CL2006 nematode model used in the Han et al. study has been reported to express only Aβ3-42, and not full-length Aβ1-42 (McColl et al., 2009). In vitro analysis demonstrates that Aβ3-42 self-aggregates like Aβ1-42, but more rapidly, forming fibrillar structures. Thus, confirmation of the new findings with a line expressing Aβ1-42 will be useful in future studies.
In summary, we believe there is much to be gained by considering the data of Han et al., as well as similar related findings, in the context of the antimicrobial protection model in which β-amyloid deposition is part of an innate immune response driven by immunochallenge. This approach may lead to alternative therapeutic strategies for effective modulation of β-amyloid generation that take into account Aβ’s normal function and do not necessarily target the peptide directly.
References:
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, Chang EB, Tanzi RE, Sisodia SS. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016 Jul 21;6:30028. PubMed.
Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016 May 25;8(340):340ra72. PubMed.
Bourgade K, Le Page A, Bocti C, Witkowski JM, Dupuis G, Frost EH, Fülöp T Jr. Protective Effect of Amyloid-β Peptides Against Herpes Simplex Virus-1 Infection in a Neuronal Cell Culture Model. J Alzheimers Dis. 2016;50(4):1227-41. PubMed.
McColl G, Roberts BR, Gunn AP, Perez KA, Tew DJ, Masters CL, Barnham KJ, Cherny RA, Bush AI. The Caenorhabditis elegans A beta 1-42 model of Alzheimer disease predominantly expresses A beta 3-42. J Biol Chem. 2009 Aug 21;284(34):22697-702. PubMed.
University of Pittsburgh School of Medicine
Gut microbial landscapes undergo a drastic change with age, and this is thought to impact age-related decline (Bischoff, 2016; Clark et al., 2015). Recently, several papers have also highlighted the importance of probiotics in suppressing chronic inflammation, enhancing immunity in humans (Ibrahim et al., 2010; Moro-Garcia et al., 2013) and in preventing insulin resistance as well as obesity in model organisms (Vincent et al., 2013). But the component of probiotics responsible for longevity is largely unknown.
In this study, Han and colleagues took an unbiased approach to identify longevity-promoting microbial factors from a library of single-gene knock-out E. coli. Using this screen as a platform, they went on to identify several E. coli mutants that use known lifespan-modulating pathways such as IGF1 and mTOR to exert their effect. However, two E.coli strains, Δlon and Δhns, did not use these conventional pathways. The authors further identified colanic acid (CA), a mannose containing extracellular polysaccharide made by several enterobacterial species, as the key molecule that extends healthspan and lifespan. Colanic acid seems to regulate mitochondrial homeostasis through fission, which in turn influences longevity.
This is a relevant study in the field given that most studies with microbial gut communities, probiotics and aging have been largely correlative. Furthermore, the study brings to the forefront the communication between microbial factors and mitochondria, which are known to have evolved from bacteria, that ultimately impacts host health.
This study sparks many exciting avenues for follow up.
1. Mechanism of function: The authors suggest that the mitochondrial unfolded protein response is required for CA-mediated longevity. However, whether hsp-6 or UPRmt dynamics are altered with age in response to CA, and if this is important for extension of lifespan, is unanswered. Other studies have shown that the mitochondrial unfolded protein response plays a role in longevity via cell-autonomous and non-cell-autonomous functions (Durieux et al., 2011). Does colanic acid exert its effect similarly? Does it change mitochondrial function with age, or substrate utilization?
2. Healthspan in other species: In the current study, colanic acid reduces amyloid toxicity and germline tumors in worms, and also activity and lifespan of D. melanogaster. Does colanic acid supplementation increase healthspan/ lifespan in mammals? If so, is supplementation required through life or intermittently? These questions will help determine the therapeutic potential of colanic acid, as well as uncover novel pathways that can be fine-tuned to extend healthy life.
References:
Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016 Jan;19(1):26-30. PubMed.
Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, Ja WW, Walker DW. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015 Sep 8;12(10):1656-67. Epub 2015 Aug 28 PubMed.
Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011 Jan 7;144(1):79-91. PubMed.
Ibrahim F, Ruvio S, Granlund L, Salminen S, Viitanen M, Ouwehand AC. Probiotics and immunosenescence: cheese as a carrier. FEMS Immunol Med Microbiol. 2010 Jun 1;59(1):53-9. Epub 2010 Feb 11 PubMed.
Moro-García MA, Alonso-Arias R, Baltadjieva M, Fernández Benítez C, Fernández Barrial MA, Díaz Ruisánchez E, Alonso Santos R, Alvarez Sánchez M, Saavedra Miján J, López-Larrea C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (Dordr). 2013 Aug;35(4):1311-26. Epub 2012 May 30 PubMed.
Vincent M, Philippe E, Everard A, Kassis N, Rouch C, Denom J, Takeda Y, Uchiyama S, Delzenne NM, Cani PD, Migrenne S, Magnan C. Dietary supplementation with Agaricus blazei murill extract prevents diet-induced obesity and insulin resistance in rats. Obesity (Silver Spring). 2013 Mar;21(3):553-61. PubMed.
Make a Comment
To make a comment you must login or register.