MRI and Machine Learning Depict Brain Aging in Health, Disease
Quick Links
The human brain follows a typical aging trajectory, but diseased brains are further along, according to a paper in the September 24 Nature Neuroscience. Scientists led by Tobias Kaufmann and Lars Westlye, University of Oslo, Norway, analyzed structural MRI scans from 45,000 people. Most were healthy, some had a neurological disorder. The scientists found that, in general, diseased brains appear older than healthy ones, revealing a gap between their predicted and their chronological ages. However, this “brain age gap” showed regional brain patterns that were unique to a given disorder. Not only did the accelerated aging patterns correlate with lower functional scores, they also appeared to be influenced by genetics.
- Machine learning found a typical pattern of brain aging in healthy people.
- In people with a neurological disorder, the brain appeared older than their chronological age.
- Genetic variation might explain some of this age gap.
“This is really novel,” said Ai-Ling Lin, University of Kentucky College of Medicine, Lexington. “As far as I know, there is no study that has used machine learning to look at lifespan brain aging and common brain disorders with such a huge dataset.”
“This study is an example of the powerful research that is only made possible by the ability to study brain scans from a very large number of people,” wrote Janine Bijsterbosch, Washington University Medical School, St. Louis, in an accompanying News and Views editorial. “The links between the brain age gap, brain disorders, and their genetics discovered by Kaufmann et al. provide a crucial first step toward gaining a clearer picture of the relationship between genetics, brain development and brain disease.”
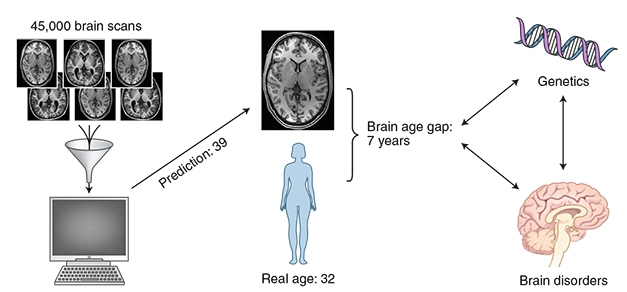
Brain Age Gap. A machine learning protocol analyzed 45,000 MRI scans from healthy brains of all ages to define a normal aging trajectory. The algorithm then predicted the ages of both healthy and diseased brains. The difference between predicted and chronological age is wider in disease and may have genetic roots. [Courtesy of Nature Neuroscience.]
The brain age gap is defined as the difference between a brain’s apparent age and its chronological age. Previous papers suggested it could be a marker of brain health, but most studies were done on a few hundred people of limited age ranges and focused on a single disorder (for a review, see Cole and Franke, 2017). In addition, they typically estimated overall brain age, neglecting disease-specific regional aging patterns.
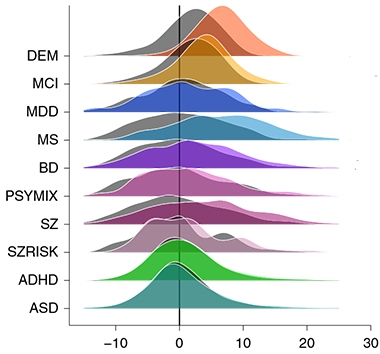
Mind the Gap. The difference in brain age gaps between people with dementia (DEM, orange) and age- and sex-matched controls (gray) is about four years; for MCI, two years. Also shown are brain age gap distributions for eight other common diseases and their respective controls. [Courtesy of Kaufmann et al., 2019. Nature.]
As part of the current study, Kaufmann and colleagues analyzed 45,615 MRI scans from people age 3–96. They used the 35,474 scans from healthy volunteers to train a machine-learning model to predict a person’s brain age. The model took into account cortical thickness, brain area, and regional volume, all visible on structural MRI. Since these features differ between the sexes, the researchers created separate models for men and women. The algorithms were then used to predict the brain ages of 5,788 people with various brain disorders, including multiple sclerosis, schizophrenia, major depressive disorder, mild cognitive impairment, and dementia. It also predicted the ages of an additional 4,353 healthy brains that were not in the training set.
The brain age gap was highest—meaning that, biologically, the brain seemed to have aged fastest—in dementia, MCI, multiple sclerosis, and schizophrenia (see image above). The brain age gap in dementia patients was about four years compared with controls. It was smaller in mixed psychoses and bipolar disorder. When the researchers analyzed brain age by region, they found aging patterns that correlated with different disorders. In schizophrenia, the frontal lobe aged fastest, and in major depressive disorder, the temporal lobe. In dementia and multiple sclerosis, it was the cerebellar-subcortical region that aged fastest. This seems counterintuitive since the cerebellum is typically spared in AD. “For technical reasons, we had to join the cerebellum and subcortical features into one joint feature set,” Kaufmann told Alzforum. “There are many more subcortical features in this joint set than cerebellar.” Some sub-cortical regions have been linked to memory loss and dementia.
The size of a person’s brain age gap correlated with functional scores specific to each disease. In dementia, wider brain age gaps were associated with lower scores on the Mini-Mental State Examination (MMSE). In multiple sclerosis, bigger gaps meant lower scores on the Expanded Disability Status Scale.
Many brain disorders are heavily influenced by genetics. Therefore, the researchers wondered whether the brain age gap also had genetic origins. The data suggested this was the case. Using single nucleotide polymorphism data from 20,170 healthy adults in the U.K. Biobank that were part of the initial MRI analysis, the researchers found that common SNPs explained 24 percent of the variance in brain age gaps in healthy controls.
When the researchers looked for variants that associated with both the brain age gap and one of 10 neurological disorders, they found 161 SNPs in total. Six were specific for dementia, and five of those lay in introns of known genes, i.e., PPP2R3A, FAM63B, IL7R, ZCWPW1, and SATB2. Bruce Yankner, Harvard Medical School, Boston, noted that SATB2 encodes a DNA binding protein that mediates attachment of DNA to the nuclear matrix. “This is interesting because a number of neurodegenerative disorders, including Alzheimer’s disease, have been associated with disruption of the nuclear lamina,” he wrote (see comment below). The five genes have not been associated previously with dementia
Next, the scientists want to know if the brain age gap shows up before symptoms of a given brain disorder, and whether findings from this study can be translated to individual patients. MRI scans are notoriously noisy, with significant variability between people, Bijsterbosch noted.
The scientists predict that studying brain age gaps identified via other imaging modalities—such as functional MRI or diffusion tensor imaging—may reveal other patterns of brain aging in disease.—Gwyneth Dickey Zakaib
References
Paper Citations
- Cole JH, Franke K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 2017 Dec;40(12):681-690. Epub 2017 Oct 23 PubMed.
Further Reading
Papers
- Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage. 2017 Oct 15;160:32-40. Epub 2017 Feb 1 PubMed.
- Zifman N, Levy-Lamdan O, Suzin G, Efrati S, Tanne D, Fogel H, Dolev I. Introducing a Novel Approach for Evaluation and Monitoring of Brain Health Across Life Span Using Direct Non-invasive Brain Network Electrophysiology. Front Aging Neurosci. 2019;11:248. Epub 2019 Sep 9 PubMed.
Primary Papers
- Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, Alnæs D, Barch DM, Baur-Streubel R, Bertolino A, Bettella F, Beyer MK, Bøen E, Borgwardt S, Brandt CL, Buitelaar J, Celius EG, Cervenka S, Conzelmann A, Córdova-Palomera A, Dale AM, de Quervain DJ, Carlo P, Djurovic S, Dørum ES, Eisenacher S, Elvsåshagen T, Espeseth T, Fatouros-Bergman H, Flyckt L, Franke B, Frei O, Haatveit B, Håberg AK, Harbo HF, Hartman CA, Heslenfeld D, Hoekstra PJ, Høgestøl EA, Jernigan TL, Jonassen R, Jönsson EG, Karolinska Schizophrenia Project (KaSP), Kirsch P, Kłoszewska I, Kolskår KK, Landrø NI, Hellard S, Lesch KP, Lovestone S, Lundervold A, Lundervold AJ, Maglanoc LA, Malt UF, Mecocci P, Melle I, Meyer-Lindenberg A, Moberget T, Norbom LB, Nordvik JE, Nyberg L, Oosterlaan J, Papalino M, Papassotiropoulos A, Pauli P, Pergola G, Persson K, Richard G, Rokicki J, Sanders AM, Selbæk G, Shadrin AA, Smeland OB, Soininen H, Sowa P, Steen VM, Tsolaki M, Ulrichsen KM, Vellas B, Wang L, Westman E, Ziegler GC, Zink M, Andreassen OA, Westlye LT. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019 Oct;22(10):1617-1623. Epub 2019 Sep 24 PubMed.
- Bijsterbosch J. How old is your brain?. Nat Neurosci. 2019 Oct;22(10):1611-1612. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Harvard Medical School
This interesting paper by Kaufmann and colleagues suggests that a number of common brain disorders are associated with potentially accelerated aging of the brain based on neuroimaging. By comparing the structure of control aging brains with those of patients, the authors compute a “brain age gap” that measures deviation from the norm. They find that the brain age gap is increased in several brain diseases, most notably in schizophrenia, multiple sclerosis, and dementia, and was associated with genetic variants that increase risk for these disorders. The central unanswered question is the mechanistic origins of the “brain age gap,” which are likely to be diverse. However, the genetic analysis of variant loci that are associated with the brain age gap reveals a discrete gene list with some loci appearing frequently. Interestingly, the most frequently detected locus is the SATB2 gene, coding a DNA binding protein that mediates attachment of DNA to the nuclear matrix. This is interesting because a number of neurodegenerative disorders, including Alzheimer’s disease, have been associated with disruption of the nuclear lamina. In addition, neuronal aging itself has been associated with reduced efficiency of nuclear-cytoplasmic transport and possible loss of nuclear membrane integrity.
A second significant locus was the SLC39A8 gene, a putative zinc transporter that has been implicated in inflammatory responses. This is intriguing in light of the evidence for a central role of inflammatory/immune responses in aging and neurological disorders, particularly Alzheimer’s disease. Hopefully future studies will determine if the age gap is a measure of parsimonious mechanisms that link neurological disorders to the biology of aging.
Make a Comment
To make a comment you must login or register.