Neuron-Glia Crosstalk Underlies Senescence, Impaired Lipid Metabolism
Quick Links
Senescence, a state in which cells neither divide nor die, but simply linger, has been linked to aging and neurodegeneration. What causes such cells to appear in old brains, and how might they wreak havoc? In the June 5 Nature, researchers led by Nancy Bonini and China Byrns at the University of Pennsylvania, Philadelphia, offered clues gleaned from flies. They found that waning mitochondrial function in neurons triggered glial senescence. In turn, these senescent glia perturbed lipid metabolism in neighboring, healthy glia, which then accumulated lipid droplets. These oily droplets came with pros and cons. When the authors blocked their formation by preventing senescence, flies lived longer and were healthier, but their brains were more susceptible to oxidative damage. Going upstream and preventing mitochondrial damage might be a more effective way to bolster brain health, the authors suggested.
- Flagging mitochondria in aging fruit fly neurons push glia into senescence.
- Senescent glia promote lipid droplets in nearby, healthy glia.
- Reining in senescence extended lifespan, but at the expense of oxidative stress.
“This is exciting work that helps tie together several independent areas of research including cellular senescence, lipid droplet formation, and mitochondrial dysfunction during aging,” Hugo Bellen, Matthew Moulton, and Lindsey Goodman at Baylor College of Medicine in Houston wrote to Alzforum. Because mitochondrial dysfunction produces reactive oxygen species, antioxidant treatment might be an effective intervention to maintain the health of aging brains, they suggested (comment below).
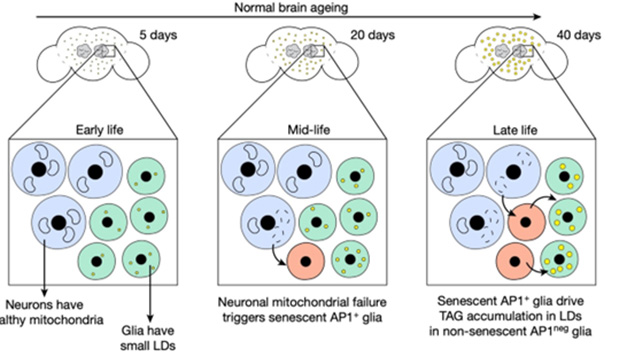
Cellular Crosstalk. In young flies (left), neurons (blue) and glia (green) are healthy, but in midlife (middle), mitochondrial damage in neurons triggers senescence in some glia (red). By late life (right), senescent glia drive lipid droplet (yellow) accumulation in healthy glia. [Courtesy of Byrns et al., Nature.]
Senescent cells appear in the mammalian brain with age, but had not been previously described in fly brain (Ito and Igaki, 2016). Byrns and Bonini earlier reported the first evidence of these cells, finding that fly glia switched on activator protein 1 (AP1), a transcription factor associated with senescence, during aging and after traumatic brain injury (Byrns et al., 2021).
To learn more about AP1-positive glia, and what they do, first author Byrns made use of a transgenic reporter line of flies that produce the fluorescent protein dsRed in cells in which AP1 is active. In young, 10-day-old reporter flies, dsRed was absent from the brain, but it appeared in glia in the antennal lobes in 20-day-old midlife flies, and in the optic lobe in late life, i.e., 40 days old.
Were these bona fide senescent cells? Byrns and colleagues isolated them and tallied their transcriptomes. This confirmed that dsRed glia had all the expected features: They were abnormally large, harbored damaged DNA, had heightened expression of metabolic and senescence-associated genes, and dampened expression of genes associated with cell division.
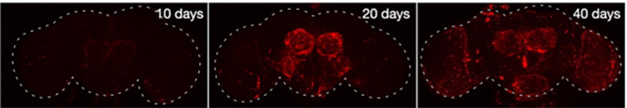
AP1 Marks Aging. Young flies (left) have no senescent cells (red) in their brains, but these gradually accumulate in mid- (middle) and late life (right). [Courtesy of Byrns et al., Nature.]
Because these senescent cells appear in the antennal and optic lobes around the time neurons begin to naturally die off there, Byrns wondered if neurodegeneration played a role, and compared neuronal gene expression in young and old flies. Older neurons produced less ATP and weakly expressed mitochondrial genes, suggesting problems with their energy supply. The authors tested this idea by using RNA interference to individually suppress genes important for these power plants, such as PINK1, OPA1, MARF, COX5A, NP15.6, ND42, and ND75. Lo and behold, each of these knockdowns made senescent glia appear in young, 10-day-old brains.
How might neuronal energy loss harm glia? Malfunctioning mitochondria are known to spark the formation of reactive oxygen species. Indeed, feeding an antioxidant to dsRed transgenic flies with damaged mitochondria prevented glial senescence. All told, the data hint that aging neurons could tip glia into senescence via oxidative stress.
Do these senescent glia affect the brain? RNA-Seq of senescent cells revealed they expressed more lipogenesis genes than did healthy glia. Co-author Gaurav Chopra at Purdue University, West Lafayette, Indiana, analyzed the cells’ lipidomes and found they accumulated about twice as much free fatty acid as did non-senescent glia. Free fatty acids can be converted to triacylglycerides, which make up lipid droplets. However, senescent cells did not form LDs; rather, these appeared in non-senescent glia, suggesting the senescent cells might be dumping lipids off to healthier cells.
In keeping with this, when the authors induced senescence in primary human fibroblasts for 10 days, then collected the media from these cultures and added it to fresh cells, those fibroblasts made lipid droplets. Not only does this suggest that secreted factors are responsible, it shows that mammalian cells can do the same, Bonini noted.
On whether blocking senescence would benefit the brain, the data were mixed. Suppressing AP1 activity for one day each week in adult flies indeed prevented lipid metabolism from going haywire, and kept LD content low. It also improved the flies’ lifespans, their climbing skills, and their ability to withstand heat, a measure of stress resistance in this species. However, flies missing AP1 altogether accumulated more oxidative damage in their brains when fed hydrogen peroxide, and, under that duress, died sooner than did controls. In addition, blocking senescence did nothing to improve neuronal mitochondrial health in normal flies.
In ongoing work, Bonini is crossing the dsRed reporter flies to models of neurodegeneration to parse what role senescent cells might play in these age-related diseases. Previous work has tied lipid dysregulation to neurodegeneration, with Chopra and Tony Wyss-Coray at Stanford University independently reporting that Aβ fibrils induce lipid droplets in microglia (Aug 2019 news; Sep 2023 news; Mar 2024 news). Chopra believes biological pathways to develop these droplets are conserved across species. “These senescent glia contributed to lipid droplet accumulation in non-senescent glia by similar lipid-related mechanisms implicated in age-onset diseases, such as Alzheimer's. This suggests that mitigating senescent glia may improve health, and opens up potential avenues for targeting aging and its diseases,” he wrote to Alzforum.—Madolyn Bowman Rogers
References
News Citations
- Newly Identified Microglia Contain Lipid Droplets, Harm Brain
- Lipid-Laden, Sluggish Microglia? Blame Aβ.
- Paper Alert: APOE4 Packs on Lipid Droplets in Microglia
Paper Citations
- Ito T, Igaki T. Dissecting cellular senescence and SASP in Drosophila. Inflamm Regen. 2016;36:25. Epub 2016 Dec 5 PubMed.
- Byrns CN, Saikumar J, Bonini NM. Glial AP1 is activated with aging and accelerated by traumatic brain injury. Nat Aging. 2021 Jul;1(7):585-597. Epub 2021 Jul 8 PubMed.
Further Reading
News
- Stirred by Tau, Neurons Amp Up Lipid Droplets in Glia
- While a Fly Sleeps, Its Glia Burn Neuronal Lipids to Refresh the Brain
- Paper Alert: APOE4 Packs on Lipid Droplets in Microglia
- Two Paths for TREM2-Positive Microglia: DAM or Senescence?
- When Autophagy Stops, Microglia Sour into Senescence
- DAMned to Death? Microglia May Proliferate to Senescence
Primary Papers
- Byrns CN, Perlegos AE, Miller KN, Jin Z, Carranza FR, Manchandra P, Beveridge CH, Randolph CE, Chaluvadi VS, Zhang SL, Srinivasan AR, Bennett FC, Sehgal A, Adams PD, Chopra G, Bonini NM. Senescent glia link mitochondrial dysfunction and lipid accumulation. Nature. 2024 Jun 5; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Baylor College of Medicine/TCH
Baylor College of Medicine
Baylor College of Medicine
This exciting work helps tie together several independent areas of research, including cellular senescence, lipid droplet formation, and mitochondrial dysfunction during aging. It has been previously reported that glia accumulate lipid droplets in response to oxidative stress in neurons (Moulton et al., 2021; Liu et al., 2015; Ioannou et al., 2019; Goodman et al., in press), and that oxidative radical levels increase with age and in AD (Grimm and Eckert, 2017; Butterfield, 2020; Peña-Bautista et al., 2019). Further, it has been hypothesized that increased glial senescence drives AD pathology, additional senescence, and the transition from MCI to AD (Lau et al., 2023).
This work provides mechanistic insight into a trigger of glial senescence via mitochondrial dysfunction in neurons. Importantly, the authors found that glial senescence markers were reduced when animals were treated with the antioxidant N-acetylcysteine amide (NACA or AD4), the same compound we and others found limits lipid peroxidation, and subsequent glial lipid droplet accumulation, as well as prevent neurodegeneration (Moulton et al., 2021; Liu et al., 2015; Goodman et al. (in press); Chung et al., 2020; Schimel et al., 2011), providing support for the use of NACA to prevent age-associated cellular toxicities.
The authors provide evidence that lipid droplets that accumulate in aged brains are sourced by lipids from the senescent glia themselves (cell autonomous), while others suggest that the source of glial lipid droplets in young brains is neurons (non-cell autonomous) (Moulton et al., 2021; Liu et al., 2015; Liu et al., 2017). Thus, it will be important to delineate the role of each cell type throughout the aging process and to understand the key impacts (protective and deleterious) that cell-autonomous and non-cell-autonomous glial lipid droplet formation may have on aging and disease. Furthermore, this pathway has been demonstrated to regulate sleep (Haynes et al., 2024), perhaps explaining sleep dysregulation seen in aging and AD patients (Goodman et al., 2024).
References:
Moulton MJ, Barish S, Ralhan I, Chang J, Goodman LD, Harland JG, Marcogliese PC, Johansson JO, Ioannou MS, Bellen HJ. Neuronal ROS-induced glial lipid droplet formation is altered by loss of Alzheimer's disease-associated genes. Proc Natl Acad Sci U S A. 2021 Dec 28;118(52) PubMed.
Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015 Jan 15;160(1-2):177-90. PubMed.
Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, Hess HF, Lippincott-Schwartz J, Liu Z. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell. 2019 May 30;177(6):1522-1535.e14. Epub 2019 May 23 PubMed.
Goodman LD, et al. Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress. Nat. Neurosci. (in press).
Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017 Apr 11; PubMed.
Butterfield DA. Brain lipid peroxidation and alzheimer disease: Synergy between the Butterfield and Mattson laboratories. Ageing Res Rev. 2020 Mar 20;:101049. PubMed.
Peña-Bautista C, López-Cuevas R, Cuevas A, Baquero M, Cháfer-Pericás C. Lipid peroxidation biomarkers correlation with medial temporal atrophy in early Alzheimer Disease. Neurochem Int. 2019 Oct;129:104519. Epub 2019 Aug 6 PubMed.
Lau V, Ramer L, Tremblay MÈ. An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer's disease. Nat Commun. 2023 Mar 25;14(1):1670. PubMed.
Chung HL, Wangler MF, Marcogliese PC, Jo J, Ravenscroft TA, Zuo Z, Duraine L, Sadeghzadeh S, Li-Kroeger D, Schmidt RE, Pestronk A, Rosenfeld JA, Burrage L, Herndon MJ, Chen S, Members of Undiagnosed Diseases Network, Shillington A, Vawter-Lee M, Hopkin R, Rodriguez-Smith J, Henrickson M, Lee B, Moser AB, Jones RO, Watkins P, Yoo T, Mar S, Choi M, Bucelli RC, Yamamoto S, Lee HK, Prada CE, Chae JH, Vogel TP, Bellen HJ. Loss- or Gain-of-Function Mutations in ACOX1 Cause Axonal Loss via Different Mechanisms. Neuron. 2020 May 20;106(4):589-606.e6. Epub 2020 Mar 12 PubMed.
Schimel AM, Abraham L, Cox D, Sene A, Kraus C, Dace DS, Ercal N, Apte RS. N-acetylcysteine amide (NACA) prevents retinal degeneration by up-regulating reduced glutathione production and reversing lipid peroxidation. Am J Pathol. 2011 May;178(5):2032-43. Epub 2011 Mar 31 PubMed.
Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, Bellen HJ. The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab. 2017 Nov 7;26(5):719-737.e6. Epub 2017 Sep 28 PubMed.
Haynes PR, Pyfrom ES, Li Y, Stein C, Cuddapah VA, Jacobs JA, Yue Z, Sehgal A. A neuron-glia lipid metabolic cycle couples daily sleep to mitochondrial homeostasis. Nat Neurosci. 2024 Feb 15; PubMed.
Goodman LD, Moulton MJ, Lin G, Bellen HJ. Does glial lipid dysregulation alter sleep in Alzheimer's and Parkinson's disease?. Trends Mol Med. 2024 Oct;30(10):913-923. Epub 2024 May 15 PubMed.
Make a Comment
To make a comment you must login or register.