New Class of BACE Inhibitor Selectively Targets APP in Cells
Quick Links
Scientists are cautiously optimistic that inhibitors of β-secretase may slow Alzheimer’s disease. The optimism comes from data that suggest BACE inhibitors safely curtail production of Aβ from its precursor, APP. The caution stems from a growing list of substrates and molecular processes that BACE inhibitors might also perturb. Can those concerns be allayed? In the March 8 Cell Reports, researchers led by Lawrence Rajendran at the University of Zurich, Switzerland, update their claim that a new class of BACE inhibitor can block cleavage of APP while allowing proteolysis of Neuregulin, L1, and perhaps other substrates to proceed as normal. Such an inhibitor could potentially treat AD without adverse side effects, the authors claim.
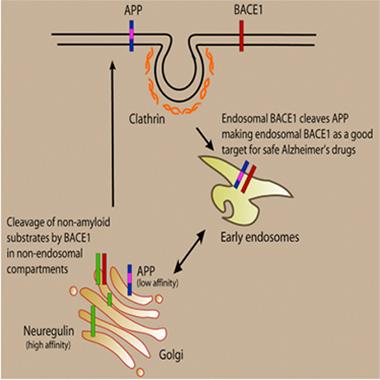
Keep ’em Separated.
Restricting BACE inhibitors to endosomes increases their specificity for blocking APP processing. [Image courtesy of Lawrence Rajendran.]
Rajendran first described an inhibitor of this kind eight years ago (see Apr 2008 news). He had hypothesized that because BACE cleaves APP in acidic endosomes, targeting an inhibitor to those organelles would spare the BACE processing that occurs on the cell surface or in other subcellular compartments. The scientists designed a sterol-based inhibitor that would be taken up by endocytosis and carried to endosomes. The compound blocked APP processing in early endosomes and prevented buildup of Aβ injected into the brains of APP/PS1 transgenic mice. But would the inhibitor spare processing of other substrates?
That’s what first authors Saoussen Ben Halima and colleagues now show in the new paper. They report that BACE processing of Neuregulin, which is required for myelination of neurons, and L1, which plays a role in axon guidance, likely occurs in the trans-Golgi network, at some physical remove from APP processing. Notably, BACE cleavage of Neuregulin/L1 occurs independently of endocytosis, they find. Hence, unlike C3, a different BACE inhibitor that spreads throughout the cell, the sterol-linked inhibitor, which enters the cell through endocytosis, had no effect on Neuregulin/L1 processing in neurons derived from human induced pluripotent stem cells.
The authors have not confirmed this substrate selectivity in animal models because the compound’s size, solubility, and pharmacodynamics make in vivo testing difficult. This means, in essence, that new derivatives of the compound have to be found to further evaluate the concept.—Tom Fagan
References
News Citations
Further Reading
News
- Wave of New BACE Inhibitors Heading to Phase 2
- Seizures in BACE1 Knockouts: Are Potassium Channels to Blame?
- Close Encounters: A New Look at Where APP and BACE1 Meet
- Does δ-Secretase Prep APP for BACE1, Boost Aβ production?
- Do BACE Inhibitors Activate Microglia?
- As DIAN Plans Trial Number Two, the Goal Is to Go Big
Primary Papers
- Ben Halima S, Mishra S, Raja KM, Willem M, Baici A, Simons K, Brüstle O, Koch P, Haass C, Caflisch A, Rajendran L. Specific Inhibition of β-Secretase Processing of the Alzheimer Disease Amyloid Precursor Protein. Cell Rep. 2016 Mar 8;14(9):2127-41. Epub 2016 Feb 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.