Newborn Neurons in the Adult Brain: Real Deal, or Glial Imposters?
Quick Links
Deep in the hippocampus, are new neurons born throughout life? Just when scientists were about to reach some consensus that the answer was yes, two recent studies disagree. In the April 5 Cell Stem Cell, Maura Boldrini and colleagues at Columbia University, New York, report that adult neurogenesis not only exists, but remains steady into old age. The researchers counted newborn neurons in samples from people aged 14–79 years, and came up with similar numbers. In the March 7 Nature, researchers led by Arturo Alvarez-Buylla at the University of California, San Francisco, reported that while neural progenitors abounded in postmortem hippocampi from prenatal or early childhood brains, they fell off the map by age 7. What gives? In older people, some of the cells that expressed markers of budding neurons turned out to be glia, the authors claim.
- Neural progenitor cells proliferate in human hippocampus throughout adulthood, says a new study.
- It blames waning angiogenesis, not faulty neurogenesis, for lost neuroplasticity in old age.
- In contrast, another paper claims brain neurogenesis fizzles during childhood.
- It claims some cells bearing neural progenitor markers are actually glia.
Who is right? Researchers who spoke with Alzforum stood squarely behind Boldrini because she used stereology, a gold standard quantitation method, to estimate numbers of neural progenitors throughout the entire dentate gyrus of postmortem brain. Alvarez-Buylla’s team estimated cell numbers using only three to five slices from each postmortem sample. “It would be very difficult to rule out neurogenesis by this method,” said Orly Lazarov of the University of Illinois in Chicago, who pointed out that because the study relied on small numbers of individuals per age group, the data could be misleading.
Others, including Jonas Frisén of the Karolinska Institute in Stockholm, pointed to a long list of previous papers supporting the existence of neurogenesis in the adult human brain. “An analogy is that 10 people go into the woods to search for blueberries,” he wrote, “Nine come back with blueberries and one does not. Are there blueberries in that forest?”
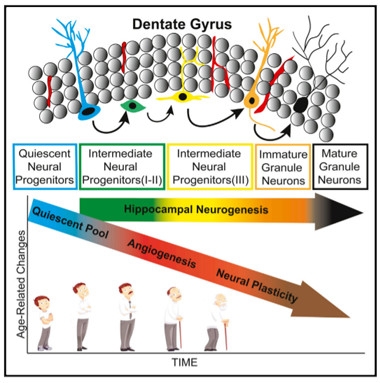
Tracking Neurogenesis.
Scientists used a combination of cell-surface markers to count neural progenitors along the development spectrum in the dentate gyrus. While neurogenesis held steady, angiogenesis and neuroplasticity declined with age. [Courtesy of Boldrini et al., Cell Stem Cell, 2018.]
Still, others acknowledged that some of those “blueberries” might have been glia. “To me, it boils down to a single question: are these proliferating cells truly neural progenitors, or not?” asked Costantino Iadecola of Weill Cornell Medical College in New York.
Numerous studies in rodents support the idea that neural progenitors in the mammalian brain continue to trickle out fresh neurons into adulthood, though factors such as aging and disease dampen the flow (Altman and Das, 1965; Sep 2001 news; Feb 2002 news; Kempermann et al., 2003; Mar 2010 news). Tracking neurogenesis in humans has been trickier, but a seminal study two decades ago set the stage: Five terminal cancer patients received an injection of bromodeoxyuridine (BrdU), a dye that incorporates into DNA during cell division. Postmortem analyses revealed evidence of dividing neurons in the dentate gyri of all of the patients (Eriksson et al., 1998). Since then, carbon-14 tracing and immunohistochemistry studies have supported the idea that new neurons arise in the adult human brain (Knoth et al., 2010; Jun 2013 news; Feb 2014 news).
Boldrini and colleagues set out to determine if age affects adult neurogenesis. They acquired hippocampal samples from 11 women and 17 men, aged 14–79, who were cognitively normal, had suffered no brain trauma, had had no microvascular pathology in the brain, and had clean toxicology reports at the time of death. The researchers collected 50-micron thick sections every 2 mm along the entirety of the hippocampus, and used both immunofluorescence and immunocytochemistry to label various cell-surface markers associated with five different stages of neural development (see image below). Finally, they estimated cell numbers throughout the dentate gyrus using stereology, whereby a computer algorithm calculates total cell numbers in a region by combining data from multiple sections. They reported the number of neural progenitors in the anterior, mid-, and posterior dentate gyrus.
The earliest neural progenitors known, called quiescent radial-like type I neural progenitors (QNPs), express GFAP, a marker shared with astrocytes; nestin, an intermediate filament protein that marks neural stem cells; and the transcription factor Sox2, which is required for the maintenance of multipotent stem cells. The researchers found that numbers of these cells decreased with age in the anterior-mid dentate gyrus. This is in keeping with the prevailing view that people are born with a finite number of these QNPs, Boldrini said.
QNPs give rise to type II intermediate neural progenitors. INPs are proliferating cells that express Ki67, a marker of actively dividing cells. Neuroblasts, or type III INPs, also proliferate, but lose expression of GFAP and Sox2. Based on expression of Ki67, nestin, and Sox2, the researchers determined that numbers of type II and III INPs remained steady, on the order of thousands of cells, in all three regions of the dentate gyrus throughout life. These neural progenitors were found in the subgranular zone (SGZ), which is proposed to be the predominant neurogenic niche in the region, as well as the granule cell layer (GCL, see image below). The findings pointed to a stable supply of neural progenitors in the dentate gyrus throughout adult life.
The researchers next asked whether those progenitors would fulfill their destiny and give rise to immature neurons and, ultimately, bona fide granule neurons. On the way to becoming fully fledged neurons, type III INPs start to express doublecortin (DCX), a microtubule-associated protein involved in neural migration. They also produce polysialylated neural cell adhesion molecule (PSA-NCAM), a glycoprotein they need for plasticity. Together, DCX and PSA-NCAM mark young neurons, which continue to express both proteins until they differentiate into mature neurons, whereupon they suppress DCX. The researchers found that the tissue donors had similar numbers of cells co-expressing DCX and PSA-NCAM, regardless of their age, suggesting neurogenesis continued unabated throughout life. Numbers of NeuN+ mature neurons also held steady, indicating that neuronal loss in the dentate gyrus is not a characteristic of healthy aging, either.
The researchers calculated that each dentate gyrus had between 10,000 and 15,000 young neurons (i.e., type III INPs and immature neurons). While the functional significance of these cell numbers is unclear, Boldrini speculated that this ongoing level of neurogenesis influences neural circuitry and cognition. For this reason, boosting neurogenesis could be a therapeutic strategy for neurodegenerative disease, she said.
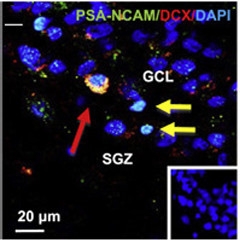
A New Neuron?
An immature neuron (red arrow) co-expressing PSA-NCAM and DCX lingers between the subgranular zone (SGZ) and the granule cell layer (GCL) in the dentate gyrus. Two other PSA-NCAM+ cells do not express DCX (yellow arrows). [Courtesy of Boldrini et al., Cell Stem Cell, 2018.]
However, while older adults appear to generate as many new neurons as younger people, those new cells may be less plastic, judging by a decline in PSA-NCAM+/DCX– cells in the anterior dentate gyrus. Curiously, using endothelial markers and stereology to measure the numbers, length, bifurcations, and total volume of capillaries, the scientists also found an age-dependent decline in angiogenesis in the same regions. The researchers proposed that a decline in angiogenesis may trigger loss of neuroplasticity without necessarily affecting neurogenesis, for example by starving new neurons of essential growth factors or nutrients.
Others were not convinced, noting that reliance on a single marker—PSA-NCAM—made the plasticity results no more than an interesting correlation. Still, Lazarov and Iadecola said the connection between age-related decline in angiogenesis and neuroplasticity was plausible. Iadecola was surprised that loss of angiogenesis did not appear to affect neurogenesis, but he noted that the donors had no obvious vascular pathology in their brains. Perhaps in people with more severe vascular problems, neurogenesis would be affected, he said.
In Grown-Up Brain, Nary a Newborn Neuron
In the Nature paper, first author Shawn Sorrells and colleagues used many of the same markers—Sox2, GFAP, DCX, and PSA-NCAM—to assess neurogenesis in postmortem samples across the lifespan. This included 11 samples from prenatal donors, the youngest of whom was only at 14 weeks gestation. They also analyzed seven samples from infants who died during their first year of life, one from a 7-year-old, one from a 13-year-old, and 17 samples from adults up to 77 years of age at the time of death. The samples came from multiple sources, and were not limited to healthy donors, or all postmortem. They included hippocampal tissue from surgical resection in 22 people with epilepsy, who ranged from three months to 64 years old.
For the postmortem samples, the researchers used three to five coronal sections to assess cell numbers. Rather than using stereology to estimate the total number of cells in the dentate gyrus, the researchers counted cells in individual sections. Three researchers independently counted each section while blinded to the age of the donor. They identified key structural landmarks, most notably the cell-dense GCL, to infer the relative locations of the cells.
In prenatal samples, the scientists found abundant proliferating Ki67+ cells that expressed the progenitor markers Sox1 and Sox2. Numbers of these cells plummeted during the first year of life, and were near zero in samples from people 7 or older. Notably, these proliferating cells never coalesced beneath the GCL to form a distinctive layer in the SGZ, a structural niche that supports neurogenesis in rodent models. The researchers confirmed the absence of this layer by electron microscopy on a subset of their samples, ranging in age from 22 gestational weeks to 48 years of age.
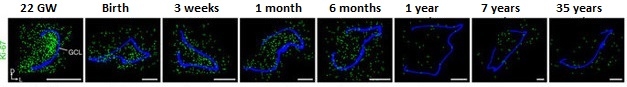
Neurogenesis Decline. Sections of dentate gyrus reveal a sharp decline in young neurons (yellow) from birth to 77 years of age. Granule cell layer traced in blue. [Courtesy of Sorrells et al., Nature, 2018.]
DCX+/PSA-NCAM+ cells, representing intermediate neural progenitors and immature neurons, clustered throughout the GCL at birth to a density of about 1,600 cells per mm2. In prenatal and infant samples, these cells had a smooth, elongated morphology characteristic of young neurons. By 13 years of age, sections only contained around two young neurons per mm2, or roughly one or two cells per section. Likewise, the investigators found no evidence of young neurons in samples from epilepsy patients older than 11. As for adults, none of the surgical or postmortem samples contained DCX+/PSA-NCAM+ cells, however the researchers did find cells that expressed PSA-NCAM without DCX. Unlike the elongated young neurons in infant samples, these cells had a more mature neuronal morphology with distinct axons and dendrites, and expressed NeuN, suggesting they were highly plastic neurons. The researchers also identified DCX+ cells in some older childhood and adult samples, but these cells co-expressed glial markers, and under the gaze of electron microscopy, had glial morphology.
The researchers also looked for evidence of neurogenesis in rhesus macaques. By staining with similar neuronal markers, they found that unlike in the human brain, proliferating neural progenitors did gather in the SGZ before birth. However, the number of these young neurons decreased eightfold between birth and 1.5 years of age, and were sparse in 7-year-old animals. Similarly, labeling dividing cells with BrdU revealed a steep drop-off in dividing neurons between 1.5 and 7 years of age.
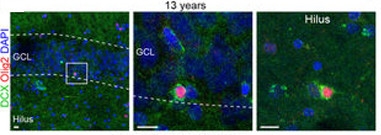
Glial Impostor?
DCX+ (green) cells co-expressing the oligodendrocyte marker Olig2 (red). [Courtesy of Sorrells et al., Nature, 2018.]
The researchers concluded that neurogenesis is robust only in the earliest stages of development, and that DCX+ cells in late childhood and adult samples were actually glia. In an email to Alzforum, Sorrells and Alvarez-Buylla speculated that the cells identified as young neurons in the Boldrini study were also likely non-neuronal. “Identifying new neurons is technically challenging—in our own recent study we made similar observations to what Boldrini et al. report, but after extensive additional analysis of the shape and appearance of the cells in question, including electron microscopy and gene expression, we determined that these cells were not in fact young neurons or neural progenitors but different types of cells altogether,” they wrote.
However, Boldrini asserted that in her study, the cells stained for both DCX and PSA-NCAM did not co-localize with cells that appeared to be glia based on the pattern of Nissl staining, and were present in the thousands. Boldrini added that the immature neurons took on a pyramidal shape, characteristic of neurons, not glia.
Sorrells and Alvarez-Buylla further drew attention to the lack of a defined layer of proliferating cells in the SGZ in their study, adding that Boldrini’s samples also appeared to lack a distinct layer of cells there. In rodents, neural progenitors gather and proliferate in the SGZ. On this issue, Boldrini thinks that perhaps in humans neurogenesis occurs in a more scattered fashion. She said that for this reason, taking stock of cells throughout the entire dentate gyrus is crucial to capture these sparse cells.—Jessica Shugart
References
News Citations
- Astrocytes Identified as Hippocampal Stem Cells
- New Neurons in Old Brains Make New Contacts
- Early Casualty, Neurogenesis Cripples Cognition in AD Mice
- Newborn Neurons Abundant in Adult Human Hippocampus
- Fresh Neurons Seed the Human Striatum Throughout Life
Paper Citations
- ALTMAN J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965 Jun;124(3):319-35. PubMed.
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003 Jan;130(2):391-9. PubMed.
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998 Nov;4(11):1313-7. PubMed.
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809. PubMed.
Further Reading
Papers
- Lazarov O, Marr RA. Of mice and men: neurogenesis, cognition and Alzheimer's disease. Front Aging Neurosci. 2013;5:43. PubMed.
- Bergmann O, Spalding KL, Frisén J. Adult Neurogenesis in Humans. Cold Spring Harb Perspect Biol. 2015 Jul 1;7(7):a018994. PubMed.
Primary Papers
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018 Apr 5;22(4):589-599.e5. PubMed.
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018 Mar 15;555(7696):377-381. Epub 2018 Mar 7 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.