Painting Pictures of Active Proteases—Probes With Potential
Quick Links
A new generation of smart fluorescent probes may help researchers monitor protease activity in real time in vivo, according to researchers at Stanford University in California. Reporting in the September 9 Nature Chemical Biology, senior author Matthew Bogyo and colleagues demonstrate how the probes light up papain-family cysteine proteases in live mice. The fluorescent probes could conceivably be used to monitor cathepsins and other proteases implicated in neurodegenerative disorders such as Alzheimer disease, suggested Bogyo in an interview with ARF, though he cautioned that the blood-brain barrier, a familiar hurdle to neuroscientists, might be the major obstacle to using these molecules for direct brain imaging.
The probes are a clever advance on suicide inhibitors, chemicals that behave as normal substrates until they are chemically altered by the target enzyme, at which point they bind tightly to the active site and inactivate the enzyme. First author Galia Blum and colleagues beefed up cathepsin suicide substrates by adding a fluorophore and a fluorescent quencher to the inhibitor’s peptide backbone. These quenched near-infrared fluorescent activity-based probes (qNIRF-ABPs) emit relatively little fluorescence in the test tube, but when modified by cathepsins, two things happen: the quencher is released and the probe binds tightly to the active site of the enzyme. The result is a fluorescent, inactive protease. A major advantage of these probes over non-quenched fluorophores is that there is little background fluorescence. “These probes allow you to look at real-time activation [of the fluorophore],” said Bogyo. “Instead of having to wait for unbound probe to flush away you can see activation within 30 minutes.” The probes are also small, stable, and fairly water soluble, which increases their versatility.
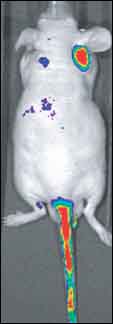
Bogyo and colleagues used the probes to non-invasively monitor cathepsin activity in mouse tumor models. Increased expression and activity of cathepsins B and L have been linked to poor outcomes in people with many types of cancer, and the proteases have become promising diagnostic and therapeutic targets. Blum and colleagues developed the quenched activity-based probes using the peptide acyloxymethylketone (AOMK) as a backbone. This peptide has been shown to be a highly specific inhibitor of cathepsins B and L. When the researchers probed mice that had been injected with two different human breast cancer cell lines, MDA-MB 231 and MDA-MB 435, significantly more probe was retained at the site of the 231 cells, which are known to have considerably more cathepsin activity (see image below).
|
|
Monitoring Tumor Cathepsin Activity in Vivo One day after injecting mice with fluorescent activity-based probes, significantly more probe is retained at the site of injection of MDA-MB 231 (red spot behind right ear), a human breast cancer cell line with high expression of cathepsins B and L. Less probe was retained by injected MDA-MB 435 cells (blue spot behind left ear). Image credit: Matt Bogyo, Stanford University |
The probes have many other potential uses. In preclinical work, they could serve to measure the efficacy of protease inhibitors in mouse models, and because they covalently bind to target proteases, they can also be used to identify those proteins in cell or tissue extracts. They could make excellent probes for PET studies and may even be useful for MR imaging, though getting sufficient contrast could be problematic, said Bogyo. He is currently working with collaborators in the Netherlands to modify the probes with quantum dots that can carry large numbers of gadolinium complexes for MR imaging. Probes targeting caspases could be used to stratify patient response to chemotherapeutic agents—Bogyo and colleagues are currently working on a family of qNIRF-ABPs that target caspases, which are activated during apoptosis. “For chemotherapy, whether or not the drugs induce apoptosis is critical,” he said.
Both caspases and cathepsins have been implicated in Alzheimer disease and other neurodegenerative disorders. Caspases are key for apoptosis, which may be linked to the neurodegenerative process (see ARF related Forum Discussion led by Yong Shen), and these proteases may play a direct role in AD by modulating APP processing (see ARF related news story). Cathepsins, as demonstrated recently by Li Gan, University of California San Francisco, and colleagues may protect against amyloid-β production and toxicity (see Mueller-Steiner et al., 2006). In fact, Gan told ARF that she is currently using these activity-based probes to monitor active cysteine proteases/cathepsins in the brains of AD animal models. The probes may also prove useful for studying the activity of other AD-related proteases, such as the aspartyl proteases β- and γ-secretase and the metalloproteases suspected of degrading Aβ, such as insulin-degrading enzyme and neprilysin. But there could be drawbacks. “Metalloproteases use water for hydrolysis of the amide bond and do not use an acyl intermediate, so the probes are harder to get to covalently attach,” said Bogyo. “This doesn’t mean that you couldn’t just use a tight binder for those targets or some kind of cross linker to get them to stick.” Getting the probes into the active site of the membrane-buried β- and γ-secretase may also be problematic.
And then, of course, there is the blood-brain barrier. “The biggest problem for imaging in the brain is delivery. The probes could potentially get there, but they are fairly big, so getting them across the blood-brain barrier could be difficult,” said Bogyo. One solution to this problem is to directly apply the probes into the brain, which would allow researchers to study protease activity in mouse models of disease. In fact, Bogyo and colleagues are currently developing the probes to directly label gliomas. The idea is that they will act as a beacon to guide surgeons to, and help them remove, brain tumors.—Tom Fagan
References
Webinar Citations
News Citations
Paper Citations
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006 Sep 21;51(6):703-14. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nature Chemical Biology. 2007 Sep 9;
Annotate
To make an annotation you must Login or Register.

Comments
University of California, San Francisco
Bogyo and colleagues developed a new generation of fluorescent quenched activity-based probes that can be used to visualize the dynamics of active proteases in whole animals. These elegant probes combine the “quenching” feature, which offers the advantage of igniting only the active protease, with the near-infrared fluorescent conjugates to reduce background in in-vivo imaging.
Accumulating evidence supports the involvement of cysteine proteases in AD pathogenesis. For example, our recent study provides evidence that cathepsin B plays a role in amyloid-β degradation and clearance, suggesting that the deficiency and dysregulation of cysteine proteases may lead to Aβ accumulation. To fully understand the improper functions of cysteine proteases in AD, we need to be able to detect the activity of specific cysteine protease at the point of action and in real time. With a proper delivery method, the probes developed by Bogyo and colleagues may be well suited to do just that.
View all comments by Li GanMake a Comment
To make a comment you must login or register.